
| A�� | ����Ʒ���Ƴ���ҺV1L��ȡ����25.00mLǡ����V2mLŨ��Ϊcmol/L����KMnO4��Һ��ȫ��Ӧ | |
| B�� | ����Ʒ�м�����H2O2���ټ�����BaCl2��Һ�����ˣ�������ϴ�ӡ��������������Ϊbg | |
| C�� | ����Ʒ������ϡ�����ַ�Ӧ���ټ�������BaCl2��Һ�����ˣ�������ϴ�ӡ��������������Ϊc g | |
| D�� | ����Ʒ������ϡ�����ַ�Ӧ�����ɵ���������ͨ��ʢ�б���NaHSO3��ϴ��ƿ��ʢ��ŨH2SO4��ϴ��ƿ��ʢ�м�ʯ�ҵĸ���ܢ�ʢ�м�ʯ�ҵĸ���ܢⶨ����ܢ�����d g |
���� A������KMnO4��Һ����������е�Na2SO3�Ļ�ѧ����ʽ������ı�KMnO4��Һ�������Լ����Na2SO3������
B��H2O2��Na2SO3�����������ƣ��ټ�����BaCl2��Һ�����ˣ������������ó����ᱵ�����ʵ������ٸ�����ԭ���غ㣬���ᱵ�����ʵ�������Na2SO3��Na2SO4����������ʵ������ٽ����Ʒag�����������Na2SO3������������
C����Ʒ������ϡ�����ַ�Ӧ������Na2SO3���ټ�������BaCl2��Һ�������Ʒ�Ӧ�������ᱵ���������ˣ�������ϴ�ӡ��������������Ϊc gΪ���ᱵ�������������غ���Լ���������Ƶ��������Ӷ����������Na2SO3������������
D����Ʒ������ϡ�����ַ�Ӧ��Na2SO3ת��Ϊ�����������壬ͨ���ⶨ��������������������������Ƶ��������Ӷ��������Na2SO3��������������������ϡ�����ַ�Ӧ�����Ȼ������壬���Բⶨ�Ľ����ȷ��
��� �⣺A��������е�Na2SO3��KMnO4��Һ����������ԭ��Ӧ�����ݵ�ʧ�����غ�������ı�KMnO4��ҺV2 mL����Na2SO3�����ʵ��������������������������Na2SO3��������������A��ȷ��
B��H2O2��Na2SO3�����������ƣ��ټ�����BaCl2��Һ�����ˣ������������ó����ᱵ�����ʵ�����Ϊ$\frac{b}{233}$mol���ٸ�����ԭ���غ㣬���ᱵ�����ʵ�������Na2SO3��Na2SO4����������ʵ���Ϊ$\frac{b}{233}$mol���ٽ����Ʒag����Na2SO3Ϊxmol��Na2SO4Ϊymol�з������
126x+142y=a
x+y=$\frac{b}{233}$�ɽ��x�����������������������Na2SO3��������������B��ȷ��
C����Ʒ������ϡ�����ַ�Ӧ������Na2SO3���ټ�������BaCl2��Һ�������Ʒ�Ӧ�������ᱵ���������ˣ�������ϴ�ӡ��������������Ϊc gΪ���ᱵ�������������غ���Լ���������Ƶ�����Ϊ$\frac{c}{233}$��142g��Na2SO3������Ϊa-$\frac{c}{233}$��142g���Ӷ����������Na2SO3��������������C��ȷ��
D����Ʒ������ϡ�����ַ�Ӧ��Na2SO3ת��Ϊ�����������壬ͨ���ⶨ��������������������������Ƶ��������Ӷ��������Na2SO3��������������������ϡ�����ַ�Ӧ�����Ȼ������壬����ͨ���������ƺ���������������Ӳ�����ȷ����D����
��ѡD��
���� ������Ҫ������Na2SO3��Na2SO4��������������ƺ����IJⶨ����Ҫ�����������ƵĻ�ԭ�����ʵ�飬����֪ʶ��Ǩ����������ƺ�����ʵ�鷽�����������Ѷ��еȣ�


 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ʹʪ�����ɫʯ����ֽ��� | B�� | ������������ˮ��ˮ��Һ�ʼ��� | ||
| C�� | �������������ܲ������� | D�� | ��ˮ���ȶ��������ֽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SiH4��CH4�ȶ� | B�� | S2-�뾶��Cl-��С | ||
| C�� | 7834Se��8034Se��Ϊͬ�������� | D�� | �ǽ����ԣ�O��N��P��Si |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��̼��ĥ�ɷ�ĩ�ɼӿ췴Ӧ���� | |
| B�� | ����̼�������ɼӿ췴Ӧ���� | |
| C�� | �����¶ȿɼӿ췴Ӧ���� | |
| D�� | �����������ʱ���������г��뺤������Ӧ���ʲ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �屽�е��嵥�ʣ�NaOH��Һ����Һ�� | B�� | �������еı������� | ||
| C�� | �Ҵ��е�ˮ��CaO������ | D�� | �����е���ϩ�����Ը��������Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�Ӱ뾶��B��A�����Ӱ뾶C��D | |
| B�� | B�ĵ�������A�������������û���Ӧ | |
| C�� | Ԫ��B��D���γ�BD2�͵Ĺ��ۻ����� | |
| D�� | D�ĵ����ж����Ҹ���ĵ���D��Ư���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | MgO��H2SO4��Na2O��CaCl2 | B�� | MnO2��HNO3��KOH��K2CO3 | ||
| C�� | SO2��NaHSO4��Ca��OH��2��KCl | D�� | CH3OH��CH3COOH��C2H5OH��CH4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ǿ������Һ�У�K+��Al3+��Cl-��SO42- | |
| B�� | ���д���NH4+����Һ��Mg2+��S2-��OH-��I- | |
| C�� | ͨ������NO2����Һ��K+��Na+��SO32-��AlO2- | |
| D�� | ǿ������Һ�У�Na+��Fe3+��NO3-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
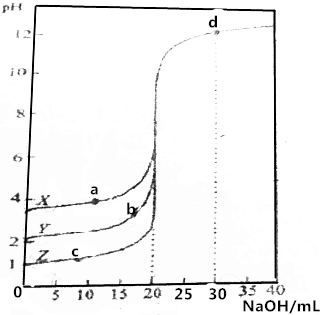 ��0.1000mol•L-1��NaOH��Һ�ֱ�ζ�0.1000mol•L-1��20.00mLX��Y��Z��������Һ����ҺpH�����NaOH���֮��Ĺ�ϵ��ͼ��ʾ������˵��������ǣ�������
��0.1000mol•L-1��NaOH��Һ�ֱ�ζ�0.1000mol•L-1��20.00mLX��Y��Z��������Һ����ҺpH�����NaOH���֮��Ĺ�ϵ��ͼ��ʾ������˵��������ǣ�������| A�� | ZΪһԪǿ�� | |
| B�� | d���c��OH-��Ϊ0.02000mol•L-1 | |
| C�� | a��b��c��b��������ӵ����ʵ���Ũ����� | |
| D�� | X��YΪһԪ���ᣬ������볣����Ka��x����Ka��Y�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com