
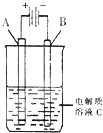
 �£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ�
�£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ�| 16g |
| 32g/mol |
| ||
| 0.56L |
| 22.4L/mol |
| 0.05mol |
| 0.5L |
| 10-14 |
| 0.1 |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
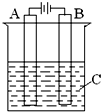 �£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬�������������ﷴӦ�������ɵ�����ˮ���������ȼ�ϣ�
�£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬�������������ﷴӦ�������ɵ�����ˮ���������ȼ�ϣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
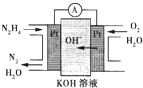 �£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г��õ�����������ش��������⣺
�£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г��õ�����������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬�������ȼ�ϣ�
�£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬�������ȼ�ϣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
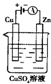 �£�N2H4���ֳ����������������ȼ�ϣ���һ����ȼ�ϵ���еĵ������Һ��20%��30%��KOH��Һ����ȼ�ϵ�ؿ���Ϊ��ͼװ���еĵ�Դ�������жϴ�����ǣ�������
�£�N2H4���ֳ����������������ȼ�ϣ���һ����ȼ�ϵ���еĵ������Һ��20%��30%��KOH��Һ����ȼ�ϵ�ؿ���Ϊ��ͼװ���еĵ�Դ�������жϴ�����ǣ��������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com