
 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
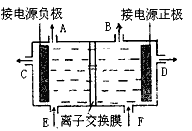
 Ŀǰ�����ϱȽ��Ƚ��ĵ���Ƽ�������ӽ���������ͼΪ���ӽ���Ĥ����ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ��
Ŀǰ�����ϱȽ��Ƚ��ĵ���Ƽ�������ӽ���������ͼΪ���ӽ���Ĥ����ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
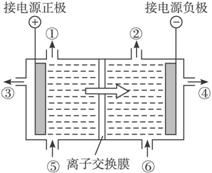
���Ʊ���ʳ��ˮ����ˮ(������NaOH)�ֱ�������������У����Եõ��������٢��Լ�NaOH��Һ������ĵ���ˮ�ӵ����е�����������������ʳ��ˮ��
����������Ϣ���ֱ�д���٢ڢۢܢݢ���ʾʲô���ʣ���___________����___________, ��___________,��___________,��___________,��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�Ƹ���ѧ����5�µڶ���ģ�⿼�ԣ������ۺϣ���ѧ���� ���ͣ������
��18�֣�Ŀǰ�����ϱȽ��Ƚ��ĵ���Ƽ�������ӽ���Ĥ����
��1�������ӽ���Ĥ����ⱥ��ʳ��ˮ�����У����Դ���������ĵ缫����������ӦΪ__________�����Դ���������ĵ缫������Һ��pH__________��ѡ����䡱�����ߡ������͡�����
��2�����������SO42�������ϸߣ����������Լ���ȥSO42�����������Լ�˳�����η�����Ӧ�����ӷ���ʽΪ______________________________��
��3���ڵ���Ƶõ�NaOH������������һ������NaCl����˱���������ι������ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ��__________����ȴ��__________����д�������ƣ���ȥNaCl��
��4�����������ӽ���Ĥ�������ӽ���Ĥ��ʯī�缫������ͼ��ʾ���ۣ����ȼҵ�е����ӽ���Ĥ����ԭ������ͨ������ⱥ��Na2SO4��Һ�ķ�������NaOH��Һ��H2SO4��Һ����缫��Ϊ__________�������������������������������������bΪ__________���ӽ���Ĥ�����������������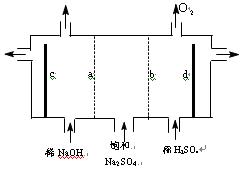
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ����5�µڶ���ģ�⿼�ԣ������ۺϣ���ѧ���� ���ͣ������
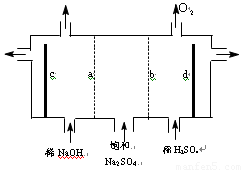
��18�֣�Ŀǰ�����ϱȽ��Ƚ��ĵ���Ƽ�������ӽ���Ĥ����
��1�������ӽ���Ĥ����ⱥ��ʳ��ˮ�����У����Դ���������ĵ缫����������ӦΪ__________�����Դ���������ĵ缫������Һ��pH__________��ѡ����䡱�����ߡ������͡�����
��2�����������SO42�������ϸߣ����������Լ���ȥSO42�����������Լ�˳�����η�����Ӧ�����ӷ���ʽΪ______________________________��
��3���ڵ���Ƶõ�NaOH������������һ������NaCl����˱���������ι������ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ��__________����ȴ��__________����д�������ƣ���ȥNaCl��
��4�����������ӽ���Ĥ�������ӽ���Ĥ��ʯī�缫������ͼ��ʾ���ۣ����ȼҵ�е����ӽ���Ĥ����ԭ������ͨ������ⱥ��Na2SO4��Һ�ķ�������NaOH��Һ��H2SO4��Һ����缫��Ϊ__________�������������������������������������bΪ__________���ӽ���Ĥ�����������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com