

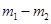 �������Ϊ
�������Ϊ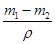 ������п��Ϊ���棬��п����Ϊ
������п��Ϊ���棬��п����Ϊ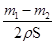 ���ʼ���п�Ʋ�ĺ�Ȳ���Ҫ����п��Ƭ��ȡ�
���ʼ���п�Ʋ�ĺ�Ȳ���Ҫ����п��Ƭ��ȡ�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
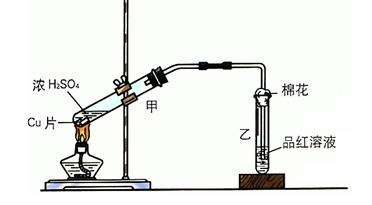
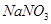 ��Һ������ͭƬ�ܽ⣬�˷�Ӧ�����ӷ���ʽΪ ��
��Һ������ͭƬ�ܽ⣬�˷�Ӧ�����ӷ���ʽΪ ��| A��40.32L | B��30.24L | C��20.16L | D��13.44L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
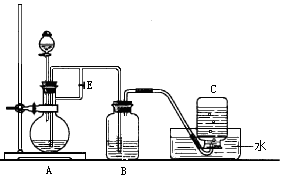
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
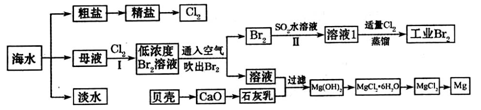
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
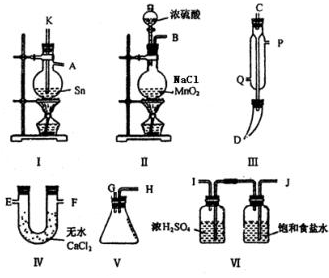
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
A����������ƽ��ȡgCuSO4��5H2O���壬�����ձ��У��� ����������ˮʹ���ܽⲢ�ָ������¡� ����������ˮʹ���ܽⲢ�ָ������¡� |
| B��������ˮϴ�ձ��Ͳ�����2��3�Σ�ÿ��ϴ��Һ��С��ע������ƿ���������� |
| C������������ƿ������ˮ����Һ���̶���1cm~2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ����ʹ���̶������С� |
| D�����Ƶõ���Һͨ��������������С�ĵ���������ƿ�С� |
 ���������˿̶��� d.������մ�����ʣ�����ʹ����������룩
���������˿̶��� d.������մ�����ʣ�����ʹ����������룩�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com