
��ѧ������ַ�����һ������Դ——����ȼ������������Ҫ�ɷ��Ǽ�����ӵĽᾧˮ����(CH4·nH2O)�����γɹ����ǣ����ں��ز���Ĵ����л�����ȱ�������У�������ϸ�����л��ʷֽ⣬����γ�ʯ�ͺ���Ȼ��������������Ȼ��������ˮ�����У��ں��ĵ������ѹ���γ������Ʊ��������壬����ǡ���ȼ���������֡���ȼ�����ľ���������(����)
A�����Ӿ��� B�����Ӿ���
C��ԭ�Ӿ��� D����������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�����Ȼ�ѧ����ʽ��
��CaCO3(s)===CaO��CO2(g)����H��177.7 kJ
��C(s)��H2O(s)===CO(g)��H2(g)
��H����131.3 kJ��mol��1
��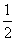 H2SO4(l)��NaOH(l)===
H2SO4(l)��NaOH(l)=== Na2SO4(l)��H2O(l)
Na2SO4(l)��H2O(l)
��H����57.3 kJ��mol��1
��C(s)��O2(g)===CO2(g)
��H����393.5 kJ��mol��1
��CO(g)��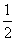 O2(g)===CO2(g)
O2(g)===CO2(g)
��H����283 kJ��mol��1
��HNO3(aq)��NaOH(aq)===NaNO3(aq)��H2O(l)
��H����57.3 kJ��mol��1
��2H2(g)��O2(g)===2H2O(l)
��H����517.6 kJ��mol��1
(1)�����Ȼ�ѧ����ʽ�У�����ȷ����_________________________________________��
����ȷ�����ɷֱ���____________________________________________________��
(2)����������Ϣ��д��Cת��ΪCO���Ȼ�ѧ����ʽ��
________________________________________________________________________��
(3)������Ӧ�У���ʾ��ȼ���ȵ��Ȼ�ѧ����ʽ��________________________________��
��ʾ�к��ȵ��Ȼ�ѧ����ʽ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D����ѧ�����Ļ���������ᴿ�Ļ���װ�á�
����ݻ���������ᴿ��ԭ�����ش�������ʵ������Ҫʹ������װ�á���A��B��C��D�����ʵ��Ŀո��С�
(1)��ȥCa(OH)2��Һ��������CaCO3__________________________________________��
(2)�ӵ�ˮ����ȡ��__________________________________________��
(3)������ˮ��ȡ����ˮ___________________________________��
(4)����ֲ���ͺ�ˮ_______________________________________��
(5)��ȥ�����е���ɳ__________________________________________��
(6)�뺣ˮɹ��ԭ���������__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ���մɡ�������ˮ��������Ϊʲô������һ��Ȼ����������ĸ�����ѡ��������ͬ���һ��(����)
A�����ά B���л����� C��������ά D�����Ͻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ʵ��Ҫ�õ�����һ�ֻ���������������Щ������������ա�
 ��
��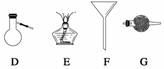
(1)������NH3��ʹ������________________________________________________��
(2)�����뻥�����ܵ�����Һ�壬��ʹ��____________________________________��
(3)������Һ̬������зе㲻ͬ����֣���ʹ��_____________________________��
(4)������������Һ���еĹ������ʣ���ʹ��_________________________________��
(5)���õ�������(CuSO4��5H2O)����ˮ����ͭ���壬��ʹ�ã�___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�йؾ������������ȷ����(����)
A����SiO2�����У���Si��O���ɵ���С��Ԫ���й���8��ԭ��
B����28 g������У���Si—Si���ۼ�����Ϊ4NA
C�����ʯ���۷е���ھ���裬����ΪC—C������С��Si—Si��
D��þ�ͺ�ͭ�ͽ����������λ����Ϊ12
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
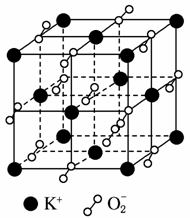
�����³������ؾ����������ṹ�����������Ļ��ϼ۲���Ϊ0�ۣ�����Ϊ��2�ۡ�����ͼ��ʾΪ�������ؾ����һ������(��������С���ظ���Ԫ)��������˵������ȷ����(����)
A���������صĻ�ѧʽΪKO2��ÿ����������4��K����4��O
B��������ÿ��K����Χ��8��O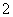 ��ÿ��O
��ÿ��O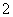 ��Χ��8��K��
����8��K��
C����������ÿ��K�����������K����8��
D�������У�0�����룭2��������Ŀ��Ϊ2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̫���ܵĿ�����������21����һ����Ҫ���⡣���ô��ܽ��ʴ���̫���ܵ�ԭ���ǣ�������̫��������ij�����ۻ��������������������ι̻��ͷų���Ӧ����������֪�±����ݣ�
| �� | �۵�(��) | �ۻ�����(kJ·mol��1) | �ο��۸�(Ԫ·t��1) |
| CaCl2·6H2O | 29.9 | 37.3 | 780��850 |
| Na2SO4·10H2O | 32.4 | 77.0 | 800��900 |
| Na2HPO4·12H2O | 35.1 | 100.1 | 1 600��2 000 |
| Na2S2O3·5H2O | 45.0 | 49.7 | 1 400��1 800 |
����������ѡ����Ϊ���ܽ��ʵ���(����)
A��CaCl2·6H2O B��Na2SO4·10H2O
C��Na2HPO4·12H2O D��Na2S2O3·5H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л���Ľṹ���á�����ʽ����ʾ����CH3—CH===CH—CH3�ɼ�дΪ���л���X�ļ���ʽΪ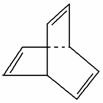 ��
��
(1)�л���Y��X��ͬ���칹�壬�����ڷ�������д��Y�Ľṹ��ʽ��____________________��
(2)Y����ϩ��һ�������·����Ӿ۷�Ӧ��д����Ӧ�Ļ�ѧ����ʽ��______________________________________________��
(3)X��������H2��һ�������·�Ӧ�����ɻ�״�ı�����Z��Z��һ�ȴ�����________�֡�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com