
ЕтдкПЦбагыЩњЛюжагаживЊгІгУЁЃФГаЫШЄаЁзщгУ0.50molЁЄL-1KIЁЂ0.2ЃЅЕэЗлШмвКЁЂ0.20molЁЄL-1K2S2O8ЁЂ0.10molЁЄL-1Na2S2O3ЕШЪдМСЃЌЬНОПЗДгІЬѕМўЖдЛЏбЇЗДгІЫйТЪЕФгАЯьЁЃ
вбжЊЃКS2O82-+2I-ЈT2SO42-+I2ЃЈТ§ЃЉЃЌI2+2S2O32-ЈTS4O62-+2I- ЃЈПьЃЉЃЌ
ЃЈ1ЃЉЯђKIЁЂNa2S2O3гыЕэЗлЕФЛьКЯШмвКжаМгШывЛЖЈСПЕФK2S2O8ШмвКЃЌЕБШмвКжаЕФNa2S2O3КФОЁКѓЃЌШмвКбеЩЋНЋгЩЮоЩЋБфГЩЮЊ ЩЋЁЃЮЊШЗБЃФмЙлВьЕНИУЯжЯѓЃЌS2O32ЁЊгыS2O82ЁЊГѕЪМЕФЮяжЪЕФСПашТњзуЕФЗЖЮЇЮЊЃКnЃЈS2O32ЁЊЃЉЃКnЃЈS2O82ЁЊЃЉ ЁЃ
ЃЈ2ЃЉЮЊЬНЬжЗДгІЮяХЈЖШЖдЛЏбЇЗДгІЫйТЪЕФгАЯьЃЌЩшМЦЕФЪЕбщЗНАИШчЯТБэЃК
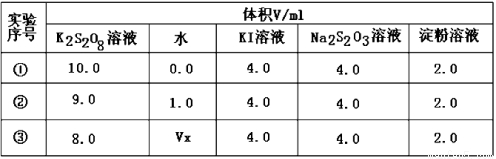
БэжаVx= mLЃЌЗДгІЫйТЪзюПьЕФЪЧ ЃЈЬюађКХЃЉЁЃ
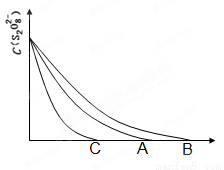
ЃЈ3ЃЉвбжЊЭМжаAЧњЯпЮЊФГЬѕМўЯТЃЌХЈЖШcЃЈS2O82ЁЊЃЉЁЋ ЗДгІЪБМфtЕФБфЛЏЧњЯпЭМЃЌШєБЃГж
ЦфЫћЬѕМўВЛБфЃЌ ЃЈЬюЁАBЁБЛђЁАCЁБЃЉЧњЯпЮЊНЕЕЭЗДгІЮТЖШЃЌ ЃЈЬюЁАBЁБЛђЁАCЁБЃЉЧњЯпЮЊМгШыДпЛЏМСЁЃ
ЃЈ1ЃЉРЖЃЌ<2
(2)2.0ЃЌЂй
ЃЈ3ЃЉBЃЛC
ЁОНтЮіЁП
ЪдЬтЗжЮіЃКЃЈ1ЃЉгЩвбжЊПЩЕУЃЌЯђKIЁЂNa2S2O3гыЕэЗлЕФЛьКЯШмвКжаМгШывЛЖЈСПЕФK2S2O8ШмвКЃЌЯШЗЂS2O82-+2I-ЈT2SO42-+I2ЃЈТ§ЃЉЃЌКѓЗЂЩњI2+2S2O32-ЈTS4O62-+2I- ЃЈПьЃЉЃЌЕБS2O32-КФОЁКѓЃЌЕтВХФмгыЕэЗлзїгУЯдЪОРЖЩЋЃЌИљОнЗНГЬЪНS2O82-+2I-ЈT2SO42-+I2жЊЃЌЩњГЩ1molЕташЮЊ1molS2O82-ЃЌИљОнI2+2S2O32-ЈTS4O62-+2I- ЗНГЬЪНжЊЃЌI2гыS2O32-ЕФЮяжЪЕФСПЕФЙиЯЕЮЊ1ЃК2ЃЌМД1molЕташ2molS2O32-ЃЌЧЁКУЗДгІЪБnЃЈS2O32-ЃЉЃКnЃЈS2O82-ЃЉ=2ЃК1ЃЌЮЊШЗБЃФмЙлВьЕНРЖЩЋЃЌЕташгаЪЃгрЃЌnЃЈS2O32-ЃЉгІЩйСПЃЌЫљвдnЃЈS2O32-ЃЉЃКnЃЈS2O82-ЃЉЃМ2ЃК1ЃЌ
ЃЈ2ЃЉИУЪЕбщЪЧЬНЬжK2S2O8ШмвКХЈЖШЕФБфЛЏЖдЗДгІЫйТЪЕФгАЯьЃЌЪЕбщЂкгыЪЕбщЂйЖдееЃЌШмвКЬхЛ§вЛжБЪЧ10mL,ЮЊШЗБЃШмвКЬхЛ§ВЛБфЃЌЫљвдVx=2.0mLЃЌЗДгІЫйТЪзюПьЕФЪЧK2S2O8ШмвКХЈЖШзюДѓЕФЂйЃЛ
ЃЈ3ЃЉЮТЖШНЕЕЭЗДгІЫйТЪМѕаЁЃЌЗДгІЪБМфМгГЄЃЌгІЮЊBЧњЯпЃЛЪЙгУДпЛЏМСЃЌЗДгІЫйТЪМгПьЃЌЗДгІЪБМфЖЬЃЌгІЮЊCЧњЯпЁЃ
ПМЕуЃКПМВщЖдгАЯьЗДгІЫйТЪЕФвђЫиЕФЗжЮіХаЖЯ


 ЕМбЇНЬГЬИпжааТПЮБъЯЕСаД№АИ
ЕМбЇНЬГЬИпжааТПЮБъЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁИпвЛЩЯбЇЦкЦкжаПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСаЙигкНКЬхЕФЫЕЗЈжае§ШЗЕФЪЧ
AЃЎНКЬхЧјБ№гкЦфЫћЗжЩЂЯЕЕФБОжЪЬиеїЪЧЖЁДяЖћЯжЯѓ
BЃЎРћгУТЫжНПЩГ§ШЅЕэЗлНКЬхжаЕФЩйСПNaCl
CЃЎFe(OH)3НКЬхДје§ЕчКЩ
DЃЎНЋжБОЖЮЊ1~100ФЩУзЕФFe3O4ПХСЃОљдШЗжЩЂЕНЫЎжаЃЌНЋЕУЕНFe3O4НКЬх
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁКўжнЪаЪєОХаЃИпвЛ12дТСЊПМЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЩшNAЮЊАЂЗќйЄЕТТоГЃЪ§ЕФжЕЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
AЃЎБъзМзДПіЯТЃЌ0ЃЎ1molCl2ШмгкЫЎЃЌзЊвЦЕФЕчзгЪ§ФПЮЊ0ЃЎ1NA
BЃЎГЃЮТГЃбЙЯТЃЌ16gO2КЭO3ЕФЛьКЯЦјЬхжаКЌгаЕФдзгзмЪ§ЮЊNA
CЃЎБъзМзДПіЯТЃЌ11.2LH2OжаКЌгаЗжзгЕФЪ§ФПЮЊ0.5NA
DЃЎ2.4gН№ЪєУОБфГЩУОРызгЪБЪЇШЅЕФЕчзгЪ§ФПЮЊ0.1NA
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁКМжнЪаИпвЛЩЯбЇЦкЦкФЉФЃФтПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСаЫЕЗЈжае§ШЗЕФЪЧЃЈ ЃЉ
AЃЎБъзМзДПіЯТЃЌ22ЃЎ4LЫЎжаЫљКЌЕФЗжзгЪ§дМЮЊ6ЃЎ02ЁС1023Иі
BЃЎ1 mol Cl2ВЮМгЗДгІзЊвЦЕчзгЪ§вЛЖЈЮЊ2 NA
CЃЎБъзМзДПіЯТЃЌaLбѕЦјКЭЕЊЦјЕФЛьКЯЮяКЌгаЕФЗжзгЪ§дМЮЊ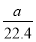 ЁС6ЃЎ02ЁС1023Иі
ЁС6ЃЎ02ЁС1023Иі
DЃЎДг1 L 0ЃЎ5 mol/LNaClШмвКжаШЁГі100 mL,ЃЌЪЃгрШмвКжаNaClЮяжЪЕФСПХЈЖШЮЊ0ЃЎ45mol/L
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁКМжнЪаИпвЛЩЯбЇЦкЦкФЉФЃФтПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
2011Фъ1дТ14ШеЃЌЮвЙњВФСЯПЦбЇЕФвЛДњзкЪІЪІВ§аїЃЌШйЛё2010ФъЖШжаЙњПЦММНчЕФзюИпШйгўЁАЙњМвзюИпПЦбЇММЪѕНБЁБЃЌЫћжївЊДгЪТИпЮТКЯН№МАИпКЯН№ИжбаОПЃЌСьЕМбажЦГіЮвЙњЕквЛДњПеаФЦјРфж§дьФјЛљИпЮТКЯН№ЮаТжвЖЦЌЕШЖрЯюГЩЙћЃЌЯТСаЙигкКЯН№ЕФа№Ъіе§ШЗЕФЪЧЃЈ ЃЉ
AЃЎКЯН№ЕФШлЕувЛАуБШзщЗжН№ЪєИп BЃЎКЯН№жажЛКЌН№ЪєдЊЫи
CЃЎКЯН№ЕФЛњаЕадФмвЛАуБШзщЗжН№ЪєКУ DЃЎКЯН№ВЛШнвзЗЂЩњЕчЛЏбЇИЏЪД
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁИпвЛЯТбЇЦкЦкжаПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЛЏбЇЗДгІN2ЃЋ3H2  2NH3ЕФФмСПБфЛЏШчЯТЭМЫљЪОЃЌИУЗДгІЕФШШЛЏбЇЗНГЬЪНЪЧЃЈ ЃЉ
2NH3ЕФФмСПБфЛЏШчЯТЭМЫљЪОЃЌИУЗДгІЕФШШЛЏбЇЗНГЬЪНЪЧЃЈ ЃЉ
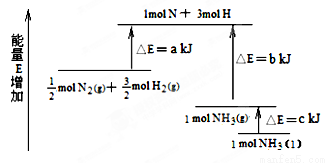
AЃЎN2(g)ЃЋ3H2(g)  2NH3(l) ЈSH ЃН2(aЁЊbЁЊc)kJЁЄmolЃ1
2NH3(l) ЈSH ЃН2(aЁЊbЁЊc)kJЁЄmolЃ1
BЃЎN2(g)ЃЋ3H2(g)  2NH3(g) ЈSH ЃН2(bЁЊa)kJЁЄmolЃ1
2NH3(g) ЈSH ЃН2(bЁЊa)kJЁЄmolЃ1
CЃЎ1/2N2(g)ЃЋ3/2H2(g)  NH3(l) ЈSH ЃН (bЃЋcЁЊa)kJЁЄmolЃ1
NH3(l) ЈSH ЃН (bЃЋcЁЊa)kJЁЄmolЃ1
DЃЎ1/2N2(g)ЃЋ3/2H2(g)  NH3(g) ЈSH ЃН (aЃЋb)kJЁЄmolЃ1
NH3(g) ЈSH ЃН (aЃЋb)kJЁЄmolЃ1
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁИпвЛЯТбЇЦкЦкжаПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСаИїЯюВЛФмгУгкБШНЯЛЏбЇЗДгІЫйТЪПьТ§ЕФЪЧЃЈ ЃЉ
AЃЎЦјЬхЕФЬхЛ§БфЛЏ BЃЎбеЩЋЕФЩюЧГБфЛЏ
CЃЎьЪБфЕФДѓаЁ DЃЎЗДгІЕФОчСвГЬЖШ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁФўВЈЪаИпвЛЩЯбЇЦкЦкФЉПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
вбжЊ 2Na2O2+2CO2=2Na2CO3+O2ЃЌвђДЫNa2O2ПЩдкКєЮќУцОпКЭЧБЫЎЭЇРязїЙЉбѕМСЁЃ
ЃЈ1ЃЉЧыдкД№ОэЩЯгУЫЋЯпЧХЗЈБъГіЩЯЪіЗНГЬЪНЕФЕчзгзЊвЦЗНЯђКЭЪ§ФПЁЃ
ЃЈ2ЃЉ ЪЧЛЙдМСЃЌ ЪЧЛЙдВњЮяЁЃ
ЃЈ3ЃЉШєзЊвЦ3molЕчзгЃЌдђЫљВњЩњЕФбѕЦјдкБъзМзДПіЯТЕФЬхЛ§ЮЊ ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁФўВЈЪаАЫаЃИпвЛЩЯбЇЦкЦкФЉСЊПМЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
гУ98%ЕФХЈСђЫсЃЈУмЖШ1.84g ЁЄ mL-1ЃЉХфжЦ100mL 1molЁЄL-1ЕФЯЁСђЫсЁЃЯжИјГіЯТСаХфжЦжаПЩФмгУЕНЕФвЧЦїЃКЂй100 mLСПЭВЃЛЂк10 mLСПЭВЃЛЂл50 mLЩеБЃЛЂмЭаХЬЬьЦНЃЛЂн100 mLШнСПЦПЃЛЂоНКЭЗЕЮЙмЃЛЂпВЃСЇАєЁЃАДЪЙгУвЧЦїЕФЯШКѓЫГађзїГіЕФЯТСаХХСажае§ШЗЕФЪЧ
AЃЎЂмЂлЂоЂпЂнЂо BЃЎЂкЂоЂлЂпЂнЂо ЁЁCЃЎЂйЂлЂнЂпЂнЂо DЃЎЂкЂлЂоЂпЂнЂо
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com