
| 1000�Ѧ�% |
| M |
| n |
| V |
| 1000�Ѧ�% |
| M |
| 1000��1.16��37.5% |
| 36.5 |


 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
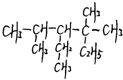 ��1����ϵͳ����������ͼ�л�������
��1����ϵͳ����������ͼ�л��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
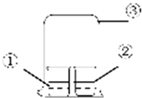 ��ͼ����ʾ��װ�ý���ʵ�飬ʵ��������Ԥ�ⲻһ�µ��ǣ�������
��ͼ����ʾ��װ�ý���ʵ�飬ʵ��������Ԥ�ⲻһ�µ��ǣ�������| ���е����� | ���е����� | Ԥ������ | |
| A | Ũ��ˮ | FeCl3��Һ | �����к��ɫ���� |
| B | Ũ��ˮ | Ũ���� | �����а��� |
| C | Ũ���� | ����KI��Һ | ������ҺΪ��ɫ |
| D | ������������Һ | ��ɫʯ����Һ | ������Һ�ȱ������ɫ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
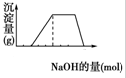 ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-�����ӣ���֪���ٸ���Һ����ɫ��Ӧû�л�ɫ���ڵ������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ����ı仯��ͼ��ʾ���ɴ˿�֪��
ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-�����ӣ���֪���ٸ���Һ����ɫ��Ӧû�л�ɫ���ڵ������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ����ı仯��ͼ��ʾ���ɴ˿�֪���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ڲⶨ����ͭ����ᾧˮ������ʵ���У����ٳ���4�� |
| B��ʹ��pH��ֽ�ⶨ��Һ��pHʱ������������ˮ��ʪpH��ֽ�������Ǵ���ģ��ⶨ�Ľ��Ҳһ���Ǵ���� |
| C������һ�����ʵ���Ũ�ȶ���Һ������ƿ��������ˮ�����ƽ����Ӱ�� |
| D������к͵ζ�ʱ����ƿ��������ˮ�Եζ������Ӱ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
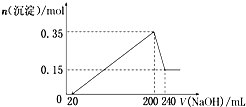 ��һ��������þ�����Ļ����Ͷ��100ml�����У�����ȫ���ܽ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ��ͼ��ʾ���������ǽ��������ᷴӦʱHCl�Ļӷ���������˵����ȷ���ǣ�������
��һ��������þ�����Ļ����Ͷ��100ml�����У�����ȫ���ܽ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ��ͼ��ʾ���������ǽ��������ᷴӦʱHCl�Ļӷ���������˵����ȷ���ǣ�������| A��þ������������Ϊ10 g |
| B��NaOH��Һ�����ʵ���Ũ��Ϊ5 mol?L-1 |
| C����������ʵ���Ũ��Ϊ5 mol?L-1 |
| D�����ɵ������ڱ�״���µ����Ϊ11.2 L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���л�ѧ�̲Ľ������ơ�þ�����������ȡ��������Ԫ�ؼ��仯�����֪ʶ����������ѧ֪ʶ�����壮
���л�ѧ�̲Ľ������ơ�þ�����������ȡ��������Ԫ�ؼ��仯�����֪ʶ����������ѧ֪ʶ�����壮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2NaOH��aq��+H2SO4��aq���TNa2SO4��aq��+2H2O��l����H=+28.7kJ/mol | ||||
| B��2NaOH��aq��+H2SO4��aq���TNa2SO4��aq��+2H2O��l����H=-28.7kJ/mol | ||||
C��NaOH��aq��+
| ||||
D��NaOH��aq��+
|
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com