
下列物质进行一氯取代反应后,只能生成四种沸点不同产物的烃是( )
|
| A. | (CH3)2CHCH2CH2CH3 | B. | (CH3CH2)2CHCH3 |
|
| C. | (CH3)2CHCH(CH3)2 | D. | (CH3)3CCH2CH3 |
| 同分异构现象和同分异构体. | |
| 专题: | 同分异构体的类型及其判定. |
| 分析: | 某烷烃发生氯代反应后,只能生成三种沸点不同的一氯代产物,则说明该有机物的一氯代物有3种;根据等效氢原子来判断各烷烃中氢原子的种类,有几种类型的氢原子就有几种一氯代物,据此进行解答. |
| 解答: | 解:A.(CH3)2CHCH2CH2CH3即为:png_iVBORw0KGgoAAAANSUhEUgAAALsAAAApCAYAAABtPlgiAAAAAXNSR0IArs4c6QAAAARnQU1BAACxjwv8YQUAAAAJcEhZcwAADsMAAA7DAcdvqGQAAAqISURBVHhe7Z0FjBTLFoYJCYEgIUFDCBBcgru7LAS/aIK7u7su7u6WAMHdg7u7E9zdnXrvO9s12yMr3Dsz2ZnpL6nsdm3PbM2pv0+dU91VE01ZWAQIltgtAgZL7G7kw4cP6sKFC1K+fPmi3rx5oz5+/Kju3bunbt68qS5fvqy+ffsm5/748UNduXJF6m/duiV1Fp7FSey/f/9WR48elfLo0SP1+fNn9fDhQ/nb3bt31blz59Tp06elIzXU0cHU//r1y6j1HrRPt5n/zzEiO3/+vLTrzJkz8rkAQZ46dUr+5k6RvX79Wg0aNEjlyJFDysyZM1XVqlXVmjVrVI8ePVTcuHFVhgwZ1IMHD+T8Fy9eyHlx4sRRDRs2lDpvcP/+fZutsAm2+vTpk60Pz549q/78+SPnvnv3zmarO3fuSJ23oC20kfYADoM+PXnypLTnxo0bUg98Bs6jn1++fGnUOmMndoQyb948lSdPHindu3dXHTp0UC1btpS/T5gwQcWPH1+lSJFCHT58WOqgRo0aKmbMmKpo0aJiOG9y+/Zt1bx5c1ub586dKz+PHTum6tSpI+0qW7as+v79u5yPEVOnTi3iGzhwoNT9V54/f6769+8v74eAKMHBwSp69Ohq+fLlIp7cuXOrgwcPGq8IAYFly5bNdiF6GkaRJk2a2GxFX9MuBF2tWjWxVaVKlWwOi/pUqVKpePHiqREjRk idN8Bp0me0sXz58mrKlCmqYMGC6tKlSypt2rTiIHr37m2creRzJEiQQCVLlkxt27bNqHXGJnaumgULFohwNLt375Y3MddVr15dzZgxwzgK4evXrypLliy2EcBb6M5btmyZUaNUu3btVLRo0dTFixdFRLTL8WpftWqVGNNd0Bl4cUf69u1rM36+fPmks8zgZXPlymUceZbr16/LCLJy5UqjRqkWLVqIrbAjYRe2wpubWbp0qapYsaJx5Hnwzlx4tAkYiXEIiRMnluOtW7eqEiVKyO9m0MGwYcOMI9fYxM4/yZQpk3EUCsKYOHGicRQi9sWLFxtHoTAk03mRgSEHI0a2hHW1MuKYr3BNly5dxFhchNmzZ5f42AwGK1eunHGkRISu/m9YZePGjcYrQ5g2bZqMbuGRP39+8fzm9xk5cqTYTYcNrsC7ml8TUdmxY4fxSnsQ+uDBg42jUDp37izhKSLHVo6sXbs2UmKnj1y1J6yiwxNHEPahQ4eMoxCePXum2rdvL79v2rTJru80bdq0UUOGDDGOXGMTO0Oqqw/rSL169VTdunUlJtVl0qRJMtwR40eGhQsXqgoVKkS6dOzY0XilPRjAldg1b9++VenSpVOjR4+2ay8jFcO1Bm/n6v+GVfCIZqZPnx6h2BmGEbz5fQoXLhyhzWfPnm33mohKt27djFfaw2d2JXYNo1+aNGnUuHHj7GzVqFEjcXARQR+5ak9YhSjCFYyA+/btM46c2b59u+Q+5jZSsO/YsWONs1xjJ3a8TETgIeggDKBL5cqVVdKkSdXjx4+Ns7wD+UR4YmcITJkypQoKCrJrb968eV2GHf+WyIidTiRfMINHzZkzp51nx2GMGTNGEjF3wgUanthJsJMnTy5OwGwrYvpatWoZZ3keHMLevXuNI2f27NmjEiVKZNdGCv2M0w2PvxY7b7xo0SLjKBQuADqKBJXhRA/ZniQiseOtGBZ1cqrZsmWLW2N2vGGVKlWMo1AIAfRwjdjNST0gfrPYCSUZjuvXry+5x4EDB6TeHUQk9qdPn7ocZZhNcgxjSLTp38iGrX9DRGJft26dyzCmdevWdp8PjdJG6rR9bWInNiTTdYRZjRUrVhhHITMveDIzCDxr1qzi2fGmdFjbtm1Vp06dnM51JyQlJIGOEEMzxafbRcxnhrDFlcH+LdioadOm0hEach08ohZ4oUKFZJ7dDGJhlNEQq86ZM0d+79mzp7ynuyD0dJXATZ06VYTOVDK2wsObIT8zh3xcwIQ2XDzM1jER4E7Sp0/vNKrhrMaPHy+/E7OXLFlSfjeDFoYOHWochcwc6jb26dNHQlqb2BEEwmEI1Zw4cULVrFnTTuwYLawE1TGT79evn0fFvnPnTgmrzB4Qo/Ah9Xw27dI3cjQkqOYOdAc4CwzO/6YgVOaDAcPHiBFDPCTTlIDxuRiYnmSa0hFGLHeKnQQSWzF3raGv8YiIHe+HrRynQRE3o7mGYx1Tly5dWnIKd4K2sN/79++NmpDkU0997t+/P8wElRHWEaZ+Ebyd2AGvzB8YRikNGjRQmzdvNv6qZIqPeXY8AGGPhoyeKSxeg7G4YULCQHLoabTgdZuZidHeqVWrVtIuRKNnZLgZgTeNHTu2JDbuhFkdhk4KU30ahlRmtIYPH25rG7ZG5NRzb8AMMyq0nflmd8JFbrYVfY2Dos+4ULEV4tfz7NzhZWqUexLMZTuC6PRI5E7mz58vEyG0kZ86FufGVoECBVSsWLFkulfDiJokSRLRmzlUHDBggHwm7YTtxA7cMeWKp5AMmCGW4kpm6DPfUSOzxhjMv+MheHMayNTaqFGjjLM8BzEkmTht1h6BDkREtIs2//z5U+rJKyZPnix1xO5RDUIZLljynvXr1xu17gOvrPtX3wDENtgDW2Ez7d0JsxAVf3Oc0kQDjNyeiNsBD08bzQ6JEYi+4wIzT/8eOXJEziOK4ALV4JxnzZol9iRMcxK7OyG88IZ39xfoNO4CJkyYUKYByXuiIogNoRP2msOiqAo3pJj5crvYif3LlCkjd7m4XU/y5mmCg0dK4uLrkGeQ2BIXr169Wh6FiGoQkpF/kEgS3pjvXkclunbtKhosXry45JzcYHS72LntvGHDBhmCvSF0qFgx6P+JqXNyYuF+rl27JnkSCS8/HWdvogrE7mgQLWo8GsZ4i1q1/lHTpk01jiwsXGOJ3SJgsMRuETBYYrcIGCyxWwQMltgtAgZL7BYBgyV2i4DBErtFwOAXYg8KqqDGjQt/SZaFhV+InefFWZtoYREefiF2C4vI4PNi5/lvvWDi1atXateuXfKMNc9l80w9z7nzxBvwPDQLKBgJzKuvLAIDnxY7wtbrISlsI8GKFZ6jZ9EIq1QaN24sT2ICj9CWKlVKVrq4e5WSRdTHZ8VOjI6QzXuMsLoFIett5li5b96TEnj0E8FbBB4+K3a20WC7CUd4fhkPzop0ttFwfN6aC6FIkSLGkUUg4bNiZ/W9K7Fr2FGAJYEsCjZvpsNCcFbFa1iErP8W3k5UFr6P34qdpDRjxoyyVwrxuS68xrzvCDsCUM9ys2bNmsmyOAv/xGfF3qtXr3DFzsr5vwlj2DiJTZ3Mq9Mt/AufFTt7nrAxjiPse6L3W2F/G1aVmyGxLVasmHEUwpIlS5z2IrHwP3xW7OwFj2dHoOxzQmFjIXbsZQaG6cbMmTM7fbsGmz6xFZ15M1ES2uPHj8sW2K52O7PwD3xW7PDkyRMJPdi2jULsrW8gkXDyDQ0kpHzRArAbGPux8E0SemtnNgjSmwLVrl3bq98wYeFdfFrswF1TdsOlmL90AGHj1a9evWrbzg1vz+aifFEB39ED7AVIuENhKzr2BLTwT3xe7P8VvD5b4lEcv6HDwp9Q6n+37Sek1D6yegAAAABJRU5ErkJgguiPgeS8mOe9kQ==,分子中含有5种位置不同的H原子,所以其一氯代物有5种,故A错误; B.(CH3CH2)2CHCH3即为:png_iVBORw0KGgoAAAANSUhEUgAAAI8AAABBCAYAAADogfdXAAAAAXNSR0IArs4c6QAAAARnQU1BAACxjwv8YQUAAAAJcEhZcwAADsMAAA7DAcdvqGQAAA1nSURBVHhe7d3nr1RVFwbw9z/wg4mJifLBGI3dKAiX2BVQwQr2rmDFCvbeW9TYu2KvYI8dewV7A3vvvff95rdkT85tCMOdueN1P8nJ3Htm5px91npW2WfWOvt/qaCgTvQJ8vz555+xVfHXX3+1e4Xu/v6voDs51SurLsnz22+/pV9//bXTl/744484udfqe7///nvs99rdiRoB55syZUq6+uqr08SJE9Onn36aXnzxxfTxxx+nu+66K914443p7rvvjs/BBx98kG666abY/9xzz3USZCNhDGRKdhlZlvk1o7qvJ8ZInw8++GDI6ZZbbkmfffZZeuaZZ2I8X3/9dcjD9thjj9XO98orr9Rk9eabb3ap107k+eSTT9JFF12Uzj///PT444+n6dOnp+effz798ssv8f+ll16aLrvssvTuu+/G5w2A4i655JJ0/fXXp59++in2NxoE+8ADD6RddtkljRw5Mm2xxRbpwAMPTG1tbem+++5Lu+66a5p77rnT7rvvHmOHJ554Ii233HKpX79+cY1VhTUS3333XbrqqqvSueeem2699dYgMdK/8cYb6fLLLw+ZPvnkk6Egynv44YfThAkTYv/7778/4yj1AWlvv/32NHr06JDTtttum/baa680YMCAMDZ6XG211dI888yTTjjhhJpM6HKhhRZKSyyxRBjgTMnjTRZ77LHHhiK22mqrtN9++6W11lorFEAASDP//POnwYMHx8XDzz//HO/PNddcaccdd0zffvtt7G8kCPiee+4Jgtxxxx0hIOMw3vnmmy+8z9tvv52GDh0aVpbhGs8555y0+eabh8U1Az/++GO68MIL0zbbbBNyJSuyHTFiRJo8eXJaYYUVgsxkm8lz3nnnpXnnnTfeY7j1AhF4Dwb21FNPhZx++OGHGAs9Io5z+szaa6/dTnecApIddthhcQ1doUYeDD/iiCPSSSedFN7DRTz00ENp0KBBaezYsbUTE4ALreKLL75Iq6++enrttddm7Jk5HOfLL7+M783KhrhV5n///fdpzJgx6aijjoqLzHDcCy64IH344Yfp9ddfT8OGDYsxOZeNpZ1++ulhfY7RHZzrm2++6XIsXW2OjbwdwePxNgcccEB8jkzJeZ111kkrr7xyEJuH32GHHdp9n/wRnJwRoN7xMJB11103PJhzZziWcfks8IbI89Zbb9VkxTvuscceIePsuTuiRp77778/rbTSSrUDghMKVazb35TDas4666xQkBBne/XVV8P1TZs2bcY3u0e2Bl5i/PjxaZ999ul2y+8LiR1JwpqOOeaYbi/MWJZccslw185lY0lIvt1228UxuoNjIpnz/9MYx40bl/bff/8IoVUFAdksv/zykV9UweKFBUZx9tlnp6233jryiixP4WyjjTYKpYOc5bTTTput8dAb8my88cZxnJmFaPpddNFFQ6a+S1Y8pBB//PHHt5N9FTXyiLMsFeu6A4FzeausskqEKB5pt912S5tuumnERtb+T3ARt912Wzr00EPTQQcdlA4++OBut/z+FVdc0Yk8zo08XVk8IM/SSy8d4zvkkENiI5jhw4d3Ig8vxBiy8pFHeHP+WRkj105+HcnDswid8pmuQBY8D5Ibk7Ha/L3YYoula665Jj6HPLM7Huf86quvQjfyJ5GjO8iJ6I9x0QtZ7bvvvkF85CEPvEB6HimjHXnWXHPNdp6nIwiZlWy44YahOAmWDVOXXXbZsBgXyv0h0ueffz7jmz0L4+BRjjvuuE5CEZoQTa7AGKpjoFz5B+/J6uGjjz4K62d1ZhvdkbEeIM8aa6wRiXpXQB7hQw5J4SeeeGJsFNi/f/9IsjsScnaQwxbjq4Z9kN/SleNPmjQpbbDBBhHOMsiBJzvyyCMjFzLBEO733HPP9MILL8T7NfJwu6uuumpNqBkURSFO7j05jxlCdTAIR1Fcr0FR7GabbRb5EyLNiQC6ggSOlZhdVS9YfkMJ7733XuQWvExHY0Ae40Mw1yBHkpOcfPLJ4U1NaTsKul4IQVKBZ599dsaev0EeLFhuIxw5bzUp5RW33HLLUNjMPMY/gb4YyhlnnNHOc7/00kvp8MMPrxnWnXfeGTOx6kwZsXgfzgFRJPc82KmnnhrekWevkYd1ELb7HxlclfwmT2sdEPtyLM4gJBZGIMiGyVyuMJFP3tOgEBdnbP42m2ApvIhxm64LW2J/Ji8iIZyxIpr9Zo1CljEyAGSaWX4wO3A+Fi1MZyCmBJVcKNf4kaeau/kbedyXmRMi+y69MjQ5Fjn5XyLMGyEL0tLxwIEDw1vn873zzjtpk002ibFxCEBmci6zQcSrkceFXHfddfHmI488EsqQNHJTU6dOjYNKjOU7FJAtmqApSsJFSD7n4t1ckh+JvT3teTJMySWIZi8sh0d0rpybuU/BSrJiEMksZ6mllgoPVLVqliQki/89NV7HefTRR0OG7pWQBbnI13g4RofwSEu2QH48w4orrhheVN4yp6DLnXbaKWZUEvGbb755xjsp7uMxJvqT32SZ4ILQKaTee++94bl4K/mZ2yTtwhYgwrXXXpvWX3/98ELcuTwmvyc+O6CZVY7j2LvzzjunxRdfPG2//fahqHyPhVINrlHkAecSchE+n8eFPv3007GxtuxJWAtPwxiq4ZSVEYwwm29+9iTkk6be7pmRUfbucizG6IbdxRdfHMQxpjPPPDNyyCFDhnSaqdULd4zJiQFl7wKcBlKTFQJnmQj75ERe0haQH5LnqFGjQv/tyAO+zH0ZNMvIcELuHRkMJN9Qohj7WK7XPDAhzEyCNTfjxmE9yFYutNgYTL4t0dOgDKSpzkgltBRGbhRjPDaftc97lNvbMKaXX3457vvJj5AHqTqRZ04hgeWi/bZkNiQMyoNaEYjvHhLr5ynNNgmop8HFT58+bY6S394ET37KKafEzE3Yy3ere5w8wgEX7URHH310u9lQq4FFIbYxyuG8NsLr8DYjRgyvpQD/NpAT2UiccwiDHicPa+ZqKaU6PfwvQ0iXAAtFfQk9Tp6CzkCaoUOHzNId+H8TCnmagEKegrpRyFNQNwp5CupGIU9B3SjkKagbhTwFdQN5hg0bWshTMPvwu9CAAf3TtGl//3LeV1DI0wT4pV4Zhh88+xIKeZqEarFXX0EhTxOgAEzxmW5NPzAqzfADshrlK6+8slaL5AdIdTL22dQZtTIKeRoMFXl6zHREKP9UOKceRoWh4jDVjgrBMnmQZpFFFokyEcVYrYxCngZBmFKVqc5acZ2SD8VdCs601SisUzGoNFQhWIaSByW1OndbtQ4qo5CnQVB7rMFQ5V0VqjB5GsQQttRaVzsnFI4pOleF2erFY4U8DYIODrXe3VUmClMK9hWU6xnjoWz673OBfq69blUU8jQIPI+yVp0HXSGTR6G7RgNhSiOlzhRtvh1bgDQaVLtcWwGFPA2CHEfDn8S4IzQEqLIUmjRRVisuhSpNidqe9MmBnAjB9I/LlVoFhTwNgs4IrUcaIKswLdedmvvkNFF2zHn0WCnMB4m3firNBFqRtXZX64h7E4U8DQKvoRly7733jtBlxqVZThOipjs9ZB5powkxN/yBrkz9WrpthT5eyfccT+jyQAJJdyugkKfB0ILkHo+HQ/Ay2pIAmZBES7Twle/zaOVeZpllItnu+HQNj8HRB9cqP3MU8jQBOjVvuOGGeM3QUIlI2Sshjk0Ton3eq97/sd/TM4S6apjrTRTytDh4JM8A8Kyc9dZbL/KoVkmaC3maAJ5i6tQpdXkMCTOP4/mEQp+7z9U28N5EIU8ToFN01KiR8fyi2UX1WQB+UFXe0So3Dwt5mgCzo0GDBgYB+hIKeZqAUoZaUDdKAXxB3SjkKagbhTwFdaOQp6BuFPIU1I1CnoK6UchTUDeUXAwe3BYk6kso5GkCLGcwfvy4KBDrSyjkaQIUdE2aNDGqCj1EW7Wgpj/PqlboZVNyoSTDr+i5cEwtUKtUDXaFQp4GAyGQZvToMfGruJ51VYLqlC0GYrEYj+63KEj+vPUzFl544Wj888T1VkUhTwPB42g1tj4GEkiYPQBbeYWuCe3EapM9fV5oy7AkgvUgcq1zq6KQp4Gw3pZFSZSPVqEfXbeoLgr9WXq1hLIMtcpKVud0va1Go5CngZCvKHDvavERBFHUbvkhi8FYGSev5Odvtc15ySSfs2aWpkDrXVWJ1pso5GkgZmWlP+QRwjwEQcOfTX+WrlHkgUyevESk1XlaYTGYQp4GAnn0q8tzOgJx5ERW+hOidESYytvkPxaW1dOeqwblPvYrSbW4WisUwRfyNBDIo4XGulpVWNvKKjKIYg2zrlb600laXZVYmPOolgUXXDBKUYWz3kYhTwOhddhsS0jSzIcAnrmjI9SDnjwpw4zKeqx55UTQEKhTworGuRUZiSwvyVPpGq1+vrdQyNNgIBCiWKfKgw+sHaqTVB6j9dgqfwsssEDMuvJNQgTp169famtri2Tbfg858FQxi6RJwqtT+95CIU8TwHtYKtIqgrpAcyjSQqMR0MYzIYlNwbw1SO1HGPkN74V8vJT38jF6E4U8/xJo27Geeev8Mp/S/wH7+4muj2qBwgAAAABJRU5ErkJgguiPgeS8mOe9kQ==,分子中含有4种位置不同的H原子,故其一氯代物有4种,故B正确; C.(CH3)2CHCH(CH3)2即为: D.(CH3)3CCH2CH3即为: 故选B. |
| 点评: | 本题考查了常见有机物的同分异构体的求算,题目难度中等,注意掌握书写同分异构体的方法,明确判断有机物分子中等效氢原子的方法:①分子中同一甲基上连接的氢原子等效;②同一碳原子所连甲基上的氢原子等效;③处于镜面对称位置(相当于平面成像时,物与像的关系)上的氢原子等效. |


 应用题点拨系列答案
应用题点拨系列答案 状元及第系列答案
状元及第系列答案 同步奥数系列答案
同步奥数系列答案科目:高中化学 来源: 题型:
肼(H2NNH2)是一种高能燃料,有关化学反应的能量变化如图所示,已知断裂1 mol化学键所需的能量(kJ):N≡N为942、O=O为500、N—N为154,则断裂1 mol N—H键所需的能量(kJ)是( )
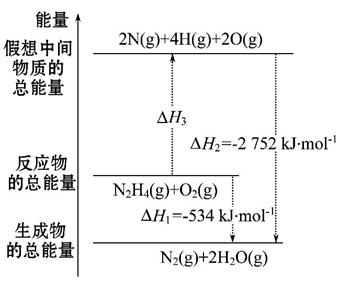
A.194 B.391 C.516 D.658
查看答案和解析>>
科目:高中化学 来源: 题型:
下列叙述正确的是( )
|
| A. | 1mol任何气体的体积一定是22.4L |
|
| B. | 标准状况下,22.4L任何气体所含分子数都约为6.02×1023个 |
|
| C. | 在标准状况下,体积为22.4L的物质都是1mol |
|
| D. | 在非标准状况下,1mol任何气体的体积不可能是22.4L |
查看答案和解析>>
科目:高中化学 来源: 题型:
下列各组有机物中,只需加入溴水就能一一鉴别的是( )
|
| A. | 己烯、苯、四氯化碳 | B. | 苯、己炔、己烯 |
|
| C. | 己烷、苯、环己烷 | D. | 甲苯、己烷、己烯 |
查看答案和解析>>
科目:高中化学 来源: 题型:
乳酸分子式为C3H6O3,在一定的条件下可发生许多化学反应,下图是采用化学方法对乳酸进行加工处理的过程,其中A、H、G为链状高分子化合物.(已知﹣OH,﹣COOH等为亲水基团,F常做内燃机的抗冻剂)请回答相关问题:
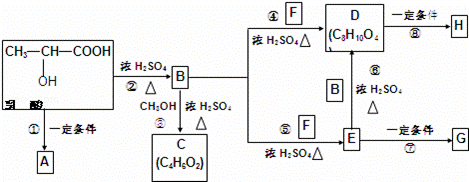
(1)推断结构简式,C: ;F: .
(2)B中所含的官能团有 ;反应③的反应类型是 .
(3)反应①的化学方程式为 .
(4)作为隐形眼镜的制作材料,对其性能的要求除应具有良好的光学性能外,还应具有良好的透气性和亲水性.一般采用E的聚合物G而不是D的聚合物H来制作隐形眼镜,其主要理由是 .
查看答案和解析>>
科目:高中化学 来源: 题型:
(1)已知胆矾失水的化学反应方程式为:CuSO4•5H2O(s)=CuSO4(s)+5H2O(l);△H=+Q1kJ/mol
(2)室温下,无水硫酸铜溶于水的热化学方程式为:
CuSO4(s)=Cu2+(aq)+SO42﹣(aq);△H=﹣Q2kJ/mol
(3)胆矾溶于水时,溶液温度降低.Q1与Q2的关系是(Q1、Q2为正数)( )
|
| A. | Q1>Q2 | B. | Q1=Q2 | C. | Q1<Q2 | D. | 无法确定 |
查看答案和解析>>
科目:高中化学 来源: 题型:
下列说法或表示方法正确的是( )
|
| A. | 等质量的硫蒸气和硫固体分别完全燃烧,后者放出的能量多 |
|
| B. | 由C(石墨)→C(金刚石)△H=+119kJ•mol﹣1可知,金刚石比石墨稳定 |
|
| C. | 在101kPa时,2gH2完全燃烧生成液态水,放出285.8kJ热量,则H2燃烧热的化学方程式表示为:2H2(g)+O2(g)=2H2O(l)△H=﹣571.6kJ•mol﹣1 |
|
| D. | 在稀溶液中,H+(aq)+OH﹣(aq)═(H2O)(l)△H=﹣57.3kJ•mol﹣1,若将含0.5mol H2SO4的浓硫酸与含1mol NaOH的溶液混合,放出的热量大于57.3kJ |
查看答案和解析>>
科目:高中化学 来源: 题型:
下列有关生活生产中的叙述合理的是( )
|
| A. | 硫酸工业中SO2转化为SO3时采用常压,是因为增大压强不会提高SO2的转化率 |
|
| B. | 打开啤酒瓶的瓶盖,有大量的气泡冒出来,该现象不能用勒夏特列原理解释 |
|
| C. | 氯碱工业中用离子交换膜电解槽电解时,往阴极室注入经过精制的饱和NaCl溶液,往阳极室注入稀氢氧化钠溶液(或去离子水) |
|
| D. | 工业上合成氨采用500℃左右的温度,最主要原因是该反应的催化剂在500℃左右时活性最好 |
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com