
���� ��1���������ָ����ˮ��Һ�������״̬���ܵ���Ļ�����ᡢ��Ρ������л�����������ﶼ�ǵ���ʣ����ʡ�����ﶼ���ǵ���ʣ�
��2�����ݼ�����ķ����жϣ�
��3��Al2��SO4��3�����Σ���ˮ����ȫ���룻����c=$\frac{n}{V}$���㣻
��4�����ӷ�ӦH++OH-=H2O����ǿ���ǿ�Ӧ���ɿ����Ե��κ�ˮ�ķ�Ӧ��
��� �⣺��1�����������ˮ��Һ������̬�¾��ܵ���Ļ���������ڵ��ʣ��ߢ�����ڻ����٢����ڷǵ���ʣ��ۢݢ����ڵ���ʣ�
�ʴ�Ϊ���ۢݢޣ�
��2���ߺ��ɫ�����������������ڽ��壬�ఱˮ ������Һ��������������ķ���Ϊ�����ЧӦ���ʴ�Ϊ�������ЧӦ��
��3��Al2��SO4��3�����Σ���ˮ����ȫ���룬����뷽��ʽΪ��Al2��SO4��3=2Al3++2SO42-��17��lg����������ˮ���250mL��Һ�����ʵ���Ũ��c=$\frac{n}{V}$=$\frac{\frac{17��lg}{342g/mol}}{0.25L}$=0.2mol/L���ʴ�Ϊ��Al2��SO4��3=2Al3++2SO42-��0.2��
��4�����ӷ�ӦH++OH-=H2O����ǿ���ǿ�Ӧ���ɿ����Ե��κ�ˮ�ķ�Ӧ����Ba��OH��2+2HCl�TBaCl2+2H2O��
�ʴ�Ϊ��Ba��OH��2+2HCl=BaCl2+2H2O��
���� ���⿼�������ӷ���ʽ��д���������ǵ���ʵ��жϡ�����μ�����������Ŀ�Ѷ��еȣ�ע���������ӷ���ʽ����дԭ����ȷ�������ǵ���ʵĸ������


 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
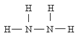 ������Ϊ�����������ȼ�ϣ�NH3��NaClO��Ӧ�ɵõ��£�
������Ϊ�����������ȼ�ϣ�NH3��NaClO��Ӧ�ɵõ��£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������ữ���ٵμ�KSCN��Һ���к�ɫ�������ɣ���ԭ��Һ��һ����Fe3+���� | |
| B�� | ������������ʹ����ʯ��ˮ����ǵ��������ɣ���ԭ��Һ��һ���д�����CO32-���� | |
| C�� | ij��Һ����ɫ��Ӧʱ����Ϊ��ɫ�������Һ��һ������Ԫ�� | |
| D�� | �ֱ���Mg2+��Cu2+��Fe2+��Na+����������Һ��ֻ��NaOH��Һ����һ���Լ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ӧ��MnO2�������� | |
| B�� | �÷�Ӧ���ڸ��ֽⷴӦ | |
| C�� | KClO3�ڷ�Ӧ��ʧȥ���� | |
| D�� | ��Ӧ��ÿ����l mol K2MnO4���������õ�2 mol���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
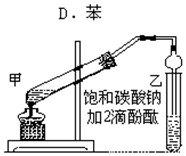 ʵ������ȡ������������Ҫ�������£�
ʵ������ȡ������������Ҫ�������£�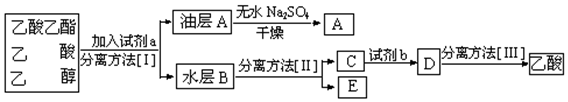
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
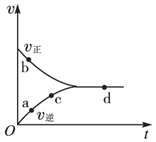 һ�������£����ܱ������г���1mol NO��1mol CO���з�Ӧ��NO��g��+CO��g��?$\frac{1}{2}$N2��g��+CO2��g������û�ѧ��Ӧ������ʱ��ı仯��ϵ��ͼ��ʾ�����д��ڻ�ѧƽ��״̬�ĵ��ǣ�������
һ�������£����ܱ������г���1mol NO��1mol CO���з�Ӧ��NO��g��+CO��g��?$\frac{1}{2}$N2��g��+CO2��g������û�ѧ��Ӧ������ʱ��ı仯��ϵ��ͼ��ʾ�����д��ڻ�ѧƽ��״̬�ĵ��ǣ�������| A�� | a�� | B�� | b�� | C�� | c�� | D�� | d�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com