
 ��ҵ�ϲ���SO2��N2��O2���������SO2������װ����ͼ��ʾ����Ӧ����װ�е�ĵ�����Һ��SO2��I2�����ķ�ӦΪ��N2��O2����I2��Ӧ����SO2+I2+2H2O�TH2SO4+2HI��
��ҵ�ϲ���SO2��N2��O2���������SO2������װ����ͼ��ʾ����Ӧ����װ�е�ĵ�����Һ��SO2��I2�����ķ�ӦΪ��N2��O2����I2��Ӧ����SO2+I2+2H2O�TH2SO4+2HI��| ����������� |
| ����������� |


 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��1 4.1���ƴ���������ϰ���������棩 ���ͣ�ʵ����
�����е�SO2�����Ϳ���������ĺ���(����g/cm3��ʾ)������Ҫ�Ŀ�������ָ�ꡣ�ڹ�ҵ�����Ϲ涨�������ж����������������ŷ�Ũ�Ȳ��ó���0.02 mg/L��
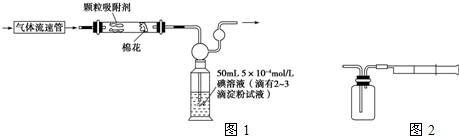
(1)Ϊ�ⶨij�ط��Ŀ�����SO2�Ϳ���������ĺ�������ͬѧ���������ͼ��ʾ��ʵ��װ�ã�

ע���������ٹ�������������λʱ����ͨ������������װ��
��Ӧ������װ�òⶨ�����е�SO2�����Ϳ���������ĺ��������ⶨ��������(��λ��cm3/min)�⣬����Ҫ�ⶨ___________________________________________________
����֪���ⵥ������ˮ��KI�����������ˮ�е��ܽ�ȡ�
����Э����ͬѧ���100 mL 5��10��4 mol/L����Һ�����ƣ�
��һ����ȷ��ȡ1.27g�ⵥ�ʼ����ձ��У�___________________________
�ڶ�����___________________________________________________________��
���������ӵڶ���������Һ�У�ȡ��10.00 mL��Һ��100 mL����ƿ�У���ˮϡ�����̶��ߡ�

(2)��ͬѧ������ͼ��ʾ����װ�òⶨ�����е�SO2������ȷ��ȡ50 mL 5��10��4 mol/L�ĵ���Һ��ע��ͼ����ʾ���ƿ�У���2��3�ε���ָʾ������ʱ��Һ����ɫ����ָ���IJⶨ�ص������ÿ�γ���100 mL��ֱ����Һ����ɫȫ���ʾ�Ϊֹ����¼��������(n)��
�ټ�����ͬѧ�IJ�����ȷ�ģ���ͬѧ�����Ĵ�������Ϊ________�Σ�����˵���õؿ����е�SO2���������ŷű���
�������ͬѧ�ø÷�������������SO2�ĺ���ʱ������õ���ֵ��ʵ�ʺ����ͣ����������ܵ�ԭ��(������Һ���ơ���������ȡ�����ֶ���������)������ֺ������裺__________________��______________________��
�۱�ͬѧ��Ϊ����ͬѧ��ʵ�鷽����Ҫ�����Ĵ���̫�࣬�����鷳���������ۺ�����������������100�����£�������ƺ����ĸĽ�������_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0102 �¿��� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(1)Ϊ�ⶨij�ؿ�����SO2�Ϳ���������ĺ�������ͬѧ���������ͼ��ʾ��ʵ��װ�ã�

��Ӧ������װ�òⶨ�����е�SO2�����Ϳ���������ĺ��������ⶨ��������(��λ��cm3/min)�⣬����Ҫ�ⶨ_________________��
����֪���ⵥ������ˮ��KI�����������ˮ�е��ܽ�ȡ�
����Э��ͬѧ���100 mL 5��10-4 mol��L-1����Һ�����ƣ�
��һ����ȷ��ȡ1.27 g�ⵥ�ʼ����ձ��У�________________��
�ڶ�����______________________________________________________________��
���������ӵڶ���������Һ�У�ȡ��10.00 mL��Һ��100 mL����ƿ�У���ˮϡ�����̶��ߡ�

(2)��ͬѧ�������м���װ�òⶨ�����е�SO2������ȷ��ȡ50 mL 5��10-4mol��L-1�ĵ���Һ��ע����ͼ��ʾ���ƿ�У���2��3�ε���ָʾ������ʱ��Һ����ɫ����ָ���IJⶨ�ص������ÿ�γ���100 mL��ֱ����Һ����ɫȫ���ʾ�Ϊֹ����¼��������(n)��
�ټ�����ͬѧ�IJ�����ȷ�ģ�������������Ϊ_____�Σ�����˵���õؿ������ŷŵ�SO2�������ϱ���
�������ͬѧ�ø÷�������������SO2�ĺ���ʱ������õ���ֵ��ʵ�ʺ����ͣ����������ܵ�ԭ��(������Һ���ơ���������ȡ�����ֶ���������)����������裺
_____________________________________________________________________��
�۱�ͬѧ��Ϊ����ͬѧ��ʵ�鷽����������̫�࣬�����鷳���������ۺ�����������������100�����£�������ƺ����ĸĽ�������_______________________________________��
(3)�ı���ʵ�����Һ����ͬѧʹ�õ�װ�û����Բⶨ��װ�ľ��ҿ����м�ȩ��Ũ�ȣ����ռ����ѡ��______________(ѡ��a.Ũ���� b.������Һ c.���Ƶ�������ͭ d.�ữ�ĸ��������Һ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�����е�SO2��������Ҫ�Ŀ�������ָ�ꡣ�ڹ�ҵ�����Ϲ涨�������ж����������������ŷ�Ũ�Ȳ��ó���0.02mg/L��
 ��ͬѧ���������ͼ��ʾ��ʵ��װ�ã��ⶨij�ؿ�����SO2�ĺ�����
��ͬѧ���������ͼ��ʾ��ʵ��װ�ã��ⶨij�ؿ�����SO2�ĺ�����
(1)������װ�òⶨ�����е�SO2�������ݵĻ�ѧ��Ӧԭ���ǣ�
�� (�û�ѧ����ʽ��ʾ)��
(2)Ӧ������װ�òⶨ�����е�SO2���������ⶨ�������٣���λ��cm3/min���⣬����Ҫ�ⶨ�������� �� ��
 ����ͬѧ��������ͼ����װ�òⶨ�����е�SO2������ȷ��ȡһ�������5��10-4mol/L�ĵ���Һ��ע����ͼ��ʾ���ƿ�У���2��3�ε���ָʾ������ʱ��Һ����ɫ����ָ���IJⶨ�ص������ÿ�γ���100mL��ֱ����Һ����ɫȫ���ʾ�Ϊֹ����¼����������n����
����ͬѧ��������ͼ����װ�òⶨ�����е�SO2������ȷ��ȡһ�������5��10-4mol/L�ĵ���Һ��ע����ͼ��ʾ���ƿ�У���2��3�ε���ָʾ������ʱ��Һ����ɫ����ָ���IJⶨ�ص������ÿ�γ���100mL��ֱ����Һ����ɫȫ���ʾ�Ϊֹ����¼����������n����
(3)����ͬѧ��ʵ��������100 mL 5��10-4mol/L�ĵ���Һ����Ҫ�õ��IJ���������
�� ��
(4) �����ͬѧ�ø÷�������������SO2�ĺ���ʱ������õ���ֵ��ʵ�ʺ����ͣ�����Ϊ���п��ܵ�ԭ������Һ���ơ���������ȡ�����ֶ����������ǣ�
�� ��
��SO2���ŷ�������������Ҫ���أ���ͬѧͨ��ʵ��̽�����ó�����pH��ʱ������Ӷ���С�Ľ��ۡ�Ϊ��һ���о�����ijɷ֣���ͬѧȡijһʱ�ε�������ˮV L������0.5mol��L-1��Ba(OH)2��Һ�����ٲ�������ʱ��ǡ������40.00 mL Ba(OH)2��Һ������㣺
(5)��V L��ˮ���ܽ�SO2������� �� mL����״������
(6)�����ɳ����������Ϊ4.50 g������ˮ�к���H2SO3�����ʵ���Ũ���Ƕ��٣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com