
̼��﮹㷺Ӧ�����մɺ�ҽҩ�������Ԧ�-﮻�ʯ����Ҫ�ɷ�ΪLi2O��Al2O3��4SiO2��Ϊԭ���Ʊ�Li2CO3�Ĺ����������£�
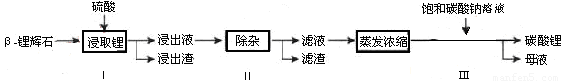
��֪��Fe3 +��Al3 +��Fe2 +��Mg2 +������������ʽ��ȫ����ʱ����Һ��pH�ֱ�Ϊ 3.2��5.2��9.7��12.4��Li2SO4��LiOH��Li2CO3��303K�µ��ܽ�ȷֱ�Ϊ34.2 g��12.7 g��1.3g��
��1������Iǰ����-﮻�ʯҪ�����ϸ������Ŀ���� ��
��2������I�У������õ���������Һ������Li+��SO42-��������Al3+��Fe3+��Fe2+��Mg2+��Ca2+��Na+�����ʣ����ڽ����¼��� ���ʯ��ʯ���� ���Ȼ��ơ���ϡ���ᡱ���Ե�����Һ��pH��6.0~6.5�����������������ӣ�Ȼ�����õ�����Һ��
��3������II�У���������H2O2��Һ��ʯ�����Na2CO3��Һ���μ������Һ�У��ɳ�ȥ�����ʽ��������� ��
��4������III�У����ɳ��������ӷ���ʽΪ ��
��5����ĸҺ�пɻ��յ���Ҫ������ ��
�����ӽӴ����ʹ��Ӧ���ͬʱ�ӿ췴Ӧ�ٶȡ�
��ʯ��ʯ��ʯ��ʯ�������ã��ſɵ�����Һ��PHֵ��������Һ��PH��6.0~6.5�����������������ӣ���ʱAl3+��Fe3+�ѳ�ȥ��
��Fe2+ ��Mg2+��Ca2+ ������������H2O2��Һ��ʯ����Ϊ�˰�Fe2+ ��Mg2+��ȥ����������Na2CO3��ҺΪ�˳�ȥCa2+ ,���˺���ҺΪLiOH��Li2SO4������Ũ����Һ�ͼ��뱥��̼������Һ�����ڵõ�Li2CO3������
��2Li++CO32-=Li2CO3��
��NaOH��Na2SO4
��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��߿���ѧ�������ר��ʮһ ����Ԫ�ؼ��仯���� ���ͣ������
��16�֣�̼��﮹㷺Ӧ�����մɺ�ҽҩ�������� -﮻�ʯ����Ҫ�ɷ�ΪLi2O
-﮻�ʯ����Ҫ�ɷ�ΪLi2O Al2O3
Al2O3 4SiO2��Ϊԭ������
4SiO2��Ϊԭ������ ��Li2CO3�Ĺ����������£�
��Li2CO3�Ĺ����������£�
��֪��Fe3+��Al3+��Fe2+��Mg2+������������ʽ��ȫ����ʱ����Һ��pH�ֱ�Ϊ3.2��5.2��9.7��12.4��Li2SO4��LiOH��Li2CO3��303K�µ��ܽ�ȷֱ�Ϊ34.2g��12.7g��1.3g��
(1)�����ǰ��B-﮻�ʯҪ�����ϸ������Ŀ����_____________��
(2)������У������õ���������Һ�к���Li+��SO42-��������Al3+��Fe3+��Fe2+��Mg2+��Ca2+��Na+�����ʣ����ڽ����¼���_____________(�ʯ��ʯ�������Ȼ��ơ���ϡ���ᡱ)�Ե�����Һ��pH��6.0~6.5�����������������ӣ�Ȼ�����õ�����Һ��
(3)����II�У���������H2O2��Һ��ʯ�����Na2CO3��Һ���μ������Һ�У��ɳ�ȥ���Ӽ�����������
_________________��
��4������III�У����ɳ��������ӷ���ʽΪ________________��
��5����ĸҺ�пɻ��յ���Ҫ������__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�켪��ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
̼��﮹㷺Ӧ�����մɺ�ҽҩ������,�Ԧ¡�﮻�ʯ(��Ҫ�ɷ�ΪLi2O��Al2O3��4SiO2)Ϊԭ���Ʊ�Li2CO3�Ĺ�����������:

��֪:Fe3+��Al3+��Fe2+��Mg2+������������ʽ��ȫ����ʱ,��Һ��pH�ֱ�Ϊ3.2��5.2��9.7��12.4;Li2SO4��LiOH��Li2CO3��303 K�µ��ܽ�ȷֱ�Ϊ34.2 g��12.7 g��1.3 g��
��1�������ǰ,�¡�﮻�ʯҪ�����ϸ������Ŀ������������������������������������������
��2���������,�����õ���������Һ�к���Li+��SO42-��������Al3+��Fe3+��Fe2+��Mg2+��Ca2+��Na+������,���ڽ����¼���������(�ʯ��ʯ�������Ȼ��ơ���ϡ���ᡱ)�Ե�����Һ��pH��6.0~6.5,����������������,Ȼ�����õ�����Һ��
��3���������,��������H2O2��Һ��ʯ�����Na2CO3��Һ���μ������Һ��,�ɳ�ȥ�����ʽ�������������������������������������������
��4���������,���ɳ��������ӷ���ʽΪ�� ��
��5����ĸҺ�пɻ��յ���Ҫ������������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�갲��ʡ�����������¿���ѧ�Ծ��������棩 ���ͣ������
̼��﮹㷺Ӧ�����մɺ�ҽҩ��������﮻�ʯ����Ҫ�ɷ�ΪLiAlSi2O6��Ϊԭ�����Ʊ�Li2CO3�Ĺ����������£�

��֪��2LiAlSi2O6+H2SO4 Li2SO4+Al2O3��4SiO2•H2O
Li2SO4+Al2O3��4SiO2•H2O
��Fe3+��Al3+��Fe2+��Mg2+������������ʽ��ȫ����ʱ����Һ��PH�ֱ�Ϊ3.2��4.7��9.0��11.1
��ijЩ���ʵ��ܽ�ȣ�S�����±�

��ش��������⣺
��1��﮻�ʯ��Ũ�����ȡ֮ǰҪ�����ϸ������Ŀ���� ��
��2����Һa�к���Li+��SO42-,������Fe3+��Al3+��Fe2+ �� Mg2+ ��Ca2+ �� Na+�����ʣ���������ڽ����¼���ʯ��ʯ�Ե�����Һ��PH��6.0��6.5����ʱ���������������� ��ʯ��ʯ������ҺPH��ԭ�������ӷ���ʽΪ ��
��3�����������Һa�м���ij��Ӽ�����Ϊ������H2O2��Һ��ʯ�����Na2CO3��Һ������������ԭ��Ӧ�����ӷ���ʽΪ ��
��4��������м��뱥��Na2CO3��Һ���˺���Ҫ����ˮϴ�ӵ�ԭ���� ��
��5������Һc�пɻ��յ���Ҫ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010����ͨ�ߵ�ѧУ����ͳһ�����������⻯ѧ���֣��㶫���� ���ͣ������
��16�֣�̼��﮹㷺Ӧ�����մɺ�ҽҩ�������� -﮻�ʯ����Ҫ�ɷ�ΪLi2O
-﮻�ʯ����Ҫ�ɷ�ΪLi2O Al2O3
Al2O3 4SiO2��Ϊԭ�����Ʊ�Li2CO3�Ĺ����������£�
4SiO2��Ϊԭ�����Ʊ�Li2CO3�Ĺ����������£�

��֪��Fe3+��Al3+��Fe2+��Mg2+������������ʽ��ȫ����ʱ����Һ��pH�ֱ�Ϊ3.2��5.2��9.7��12.4��Li2SO4��LiOH��Li2CO3��303K�µ��ܽ�ȷֱ�Ϊ34.2g��12.7g��1.3g��
(1)�����ǰ��B-﮻�ʯҪ�����ϸ������Ŀ����_____________��
(2)������У������õ���������Һ�к���Li+��SO42-��������Al3+��Fe3+��Fe2+��Mg2+��Ca2+��Na+�����ʣ����ڽ����¼���_____________(�ʯ��ʯ�������Ȼ��ơ���ϡ���ᡱ)�Ե�����Һ��pH��6.0~6.5�����������������ӣ�Ȼ�����õ�����Һ��
(3)����II�У���������H2O2��Һ��ʯ�����Na2CO3��Һ���μ������Һ�У��ɳ�ȥ���Ӽ�����������
_________________��
��4������III�У����ɳ��������ӷ���ʽΪ________________��
��5����ĸҺ�пɻ��յ���Ҫ������__________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com