
�±���Ԫ�����ڱ���һ���֣�����Ҫ��ش��������⡣
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 2 | | | | E | H | F | I | |
| 3 | A | C | D | | | | G | R |
| 4 | B | | | | | | | |
��1��Ar ��2��NaOH ��3�� ��
��
��4��Cl2+2OH- = Cl- + ClO- + H2O
(5)2Na2O2+2CO2=2Na2CO3+O2��
���������������Ԫ�����ڱ���֪��A:Na B:K C:Mg D: Al E:C F:O G:Cl H:N I:F R:Ar
��1��ArΪ�������壬�����
��2��A ��C ��D����Ԫ�طֱ��Ӧ��ˮ����Ϊ NaOH�� Mg(OH)2��Al(OH)3����NaOH������ǿ��
��3�� ������Ϊ���ӻ������Ԫ����ɫ��ӦΪ��ɫ��
������Ϊ���ӻ������Ԫ����ɫ��ӦΪ��ɫ��
��4��B������������Ӧ��ˮ����Ϊǿ���ȫ���롣�ʷ�Ӧ����ʽ ΪCl2+2OH- = Cl- + ClO- + H2O
��5��A��F�γ�Na2O��Na2O2�������ʣ����к��߸��ȶ���
���㣺�ص㿼��Ԫ�����ڱ���Ԫ�������ɣ����������ӷ�Ӧ����ʽ����д������ʽ����д�ȷ��档


 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ԫ�����ڱ��У�ͬһ����Ԫ�ػ�ѧ�������ơ�ĿǰҲ������ЩԪ�صĻ�ѧ���ʺ��������ڱ������Ϸ������·�����һ����Ԫ���������ƣ����Ϊ�Խ��߹��ݴ���ش�
��1����ڿ�����ȼ�գ�������______________�⣬Ҳ��������________________��
��2������֪��ӦBe2C��4H2O��2Be(OH)2��CH4������Al4C3������ǿ����Һ��Ӧ�����ӷ���ʽΪ_____________________________________________________________��
��3����ѧ��֤ʵ��BeCl2�ǹ��ۻ�����������һ����ʵ��֤������ʵ�鷽���ǣ�____________________________________________________________________________��
�õ���ʽ��ʾBeCl2���γɹ��̣�______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F��G��H��Ԫ�����ڱ�ǰ�����ڳ���Ԫ�أ���ԭ���������������������Ϣ���±���
| Ԫ�� | �����Ϣ |
| A | ԭ�Ӻ�����6�ֲ�ͬ�˶�״̬�ĵ��� |
| C | ��̬ԭ����s����������p����������� |
| D | ԭ�Ӱ뾶��ͬ����Ԫ������� |
| E | ��̬ԭ�����������Ų�ʽΪ3s23p1 |
| F | ��̬ԭ�ӵ������p������������ӵ������������������ӵ����������෴ |
| G | ��̬ԭ�Ӻ�����7���ܼ���������ߵ��ܼ�����6������ |
| H | ���ҹ�ʹ������ĺϽ��е�����ҪԪ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��ij����������Ԫ�ص�ԭ��M������һ����������Dz�,����ԭ�ӵ��������� ___________��д��������Χ�����Ų�ͼ___________��
��2��VIA���.��(Se).�ڻ������г����ֳ���������̬, H2SeO4��H2SeO3����_-___( ��ǿ����)��H2Se�����Ա�H2S__________(�ǿ��������)��
��3������A�Ļ�ѧʽΪNH5����������ԭ�ӵ�����㶼������Ӧϡ������ԭ�ӵ�������Ӳ�ṹ���������й�˵������ȷ���� ( )
| A��NH5�м������Ӽ����й��ۼ� |
| B��NH5���ۡ��е����NH3 |
| C��NH5����Ͷ������ˮ�У��ɲ����������� |
| D��0.1 mol NH5���5 mol N��H�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��12�֣�U��V��X��Y��Z���ֶ�����Ԫ�أ�ԭ��������������U��V��Ԫ����������������֮�;�Ϊ0��Y��Uͬ���壻X��Z�ֱ��ǵؿ��к�����ߵķǽ���Ԫ�غͽ���Ԫ�ء�
��ش��������⣺
��1������Ԫ��ԭ�Ӱ뾶�ɴ�С��˳���ǣ���ʵ��Ԫ�ط��ű�ʾ��________________��
��2����������Ԫ���еļ�����ɵĻ�����A��B��C��D������ת����ϵ��
����C������ˮ�����Ե����壻D�ǵ���ɫ���塣д��C��D��Ӧ�Ļ�ѧ����ʽ��___________________;
�����BΪ���Բ������A�Ļ�ѧʽΪ_________��Aת��ΪB�����ӷ���ʽΪ��__ __________;
�����B������Ԫ���������Һ�Լ��ԣ���B���еĻ�ѧ��������_______��A�����������Ϊ_ ___________����ѡ����ţ���a���� b���� c���� d��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ��������������B��Cͬ���ڣ�A��Dͬ���塣A��C���γ�����Һ̬��������ң�ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1��E �ǵؿ��к�����ߵĽ���Ԫ�ء�����������Ϣ�ش��������⣺
�żס����������к��зǼ��Թ��ۼ������ʵĵ���ʽ��_________��E���ӵĵ����Ų�ʽΪ____________��Cԭ�ӵĵ����Ų�ͼΪ ��Dԭ�ӵ�ԭ�ӽṹʾ��ͼΪ_______ ��
��2��B���⻯��ķе����ͬ����Ԫ���⻯��ķе㣬ԭ����_____________�����⻯��ĵ���ʽΪ_______������ԭ�ӵ��ӻ���ʽΪ _______�����ӵ����幹��Ϊ_______��
��3��DCA��E������������ˮ�������Ӧ�����ӷ���ʽ ��
��4�����ݶԽ��߹���Be��E�������ƣ�д��Be��DCA��Һ��Ӧ�����ӷ���ʽ______________________
��5����Ҫ˵���ǽ���Ԫ��X��Ԫ��Y��X��Y��Ϊ��ϡ������Ԫ�أ��ķǽ�����ǿ�������з�����ȷ����__________������ţ���
��X���ʿ���Y�����⻯�����û�����
��Xԭ�ӵ�������������Yԭ�ӵ�������������
��X���������ˮ��������Ա�Y���������ˮ���������ǿ
����H2����ʱX���ʱ�Y��������
�ݵ縺�ԣ�X>Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z��W��R�Ƕ�����Ԫ�أ�ԭ��������������Xԭ�Ӻ�����������֮��Ϊ1��2�� Yԭ�Ӻ�Zԭ�ӵĺ��������֮��Ϊ20��W��R��ͬ��������Ԫ�أ�Y���������R������������γ����ꡣ
��ش��������⣺
��1��Ԫ��X�����������ĵ���ʽΪ ��Ԫ��Z�����ӽṹʾ��ͼΪ ��
��2������ͭ��Ԫ��Y������������Ӧˮ�����ϡ��Һ������Ӧ�Ļ�ѧ����ʽΪ ��
��3��Ԫ��Wλ�����ڱ��ĵ� �壬 ��ǽ����Ա�Ԫ��R������ԭ�ӽṹ��֪ʶ����ԭ
�� ��
��4��R��һ����������ʹƷ����Һ��ɫ����ҵ����Y����̬�⻯���ˮ��Һ�������ռ���д�����ռ�
�������������ﷴӦ�����ӷ���ʽ ��
��5��Y��Z��ɵĻ�����ZY�������������������Ԫ������ҵ����Z�������X���ʺ�Y������
�������Ʊ�ZY������Z���������X���ʵ����ʵ���֮��Ϊ1��3����÷�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F�ܱ�ǰ�������еij���Ԫ�أ��������Ϣ���±���
| Ԫ�� | �����Ϣ |
| A | A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�� |
| B | BԪ�ص�ԭ�Ӽ۵����Ų�Ϊns11np14 |
| C | M�Ļ�̬ԭ��L���������K���������3�� |
| D | D�ǵ��������е�һ��������С��Ԫ�� |
| E | E�ǵؿ��к������Ľ���Ԫ�� |
| F | �ж��ֻ��ϼۣ���ij�ָ������ӵļ۵��Ӿ��н��ȶ��İ�����ṹ |
 ����
����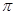 �������֮��Ϊ ��
�������֮��Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±�ΪԪ�����ڱ���һ���֡���ش��������⣺
����Ԫ�������ڱ��е����λ�ÿ�֪�١���ֱ���H��C��N��O��Mg��Al��Cl��Ca��Mn��Fe��
��1������Ԫ���У�����s������____________(��Ԫ�ط���)��
��2��д��Ԫ�آܵĻ�̬ԭ�ӵļ۵����Ų�ͼ____________________��
��3��Ԫ�ص�һ������Ϊ��________�� (����ڡ���С�ڡ�)��
��4��Ԫ�آ���̬�⻯���VSEPRģ��Ϊ________���÷���Ϊ________����(����ԡ��Ǽ��ԡ�)��������ͭ��Һ����μ�����ˮ��Һ���ɹ۲쵽������Ϊ_____________________________��
��5�����ʵľ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��
����֪��ԭ�Ӱ뾶Ϊdcm��NA���������ӵ�������Ԫ�آ����ԭ������ΪM����ش𣺾����Т�ԭ�ӵ���λ��Ϊ ,�þ�����ܶ�Ϊ ������ĸ��ʾ��
��6��ʵ��֤�����ݺ͢�������KCl��TiN��4�־���Ľṹ��NaCl����ṹ���ƣ�����ͼ��ʾ������֪3�����Ӿ���ľ������������±���
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ��mol��1 | 786 | 715 | 3401 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com