

| n |
| V |
| 0.05mol |
| 10mol/L |


 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������������������۾��IJ��ϣ���д�������йط�Ӧ�Ļ�ѧ����ʽ��
�������������������۾��IJ��ϣ���д�������йط�Ӧ�Ļ�ѧ����ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͼ��ʾ������ת����ϵ�У�A�dz����ļ�����̬�⻯�B����ʹ�����ǵ�ľ����ȼ����ɫ��ζ���壬E����Է���������D��17��G�����ڽ������˳�������Ϻ�ɫ�������ʣ������ַ�Ӧ��������û��ȫ���г�����Ӧ����δ�г�����ش��������⣺
����ͼ��ʾ������ת����ϵ�У�A�dz����ļ�����̬�⻯�B����ʹ�����ǵ�ľ����ȼ����ɫ��ζ���壬E����Է���������D��17��G�����ڽ������˳�������Ϻ�ɫ�������ʣ������ַ�Ӧ��������û��ȫ���г�����Ӧ����δ�г�����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������λ�ڶ����ڵ��������ڡ���������ķǽ���Ԫ��X��Y����֪��Ԫ������������ˮ�����Ϊǿ�ᣮ������ͼת����ϵ����Ӧ���������ֲ�������ȥ�����ش��������⣺
������λ�ڶ����ڵ��������ڡ���������ķǽ���Ԫ��X��Y����֪��Ԫ������������ˮ�����Ϊǿ�ᣮ������ͼת����ϵ����Ӧ���������ֲ�������ȥ�����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
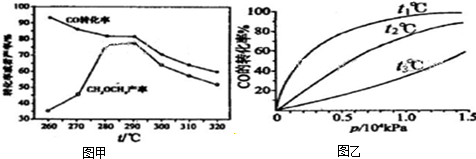
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����0.1mol?L-1 NaHCO3��Һ�У�c��OH-��+c��CO32-��=c��H+��+c��H2CO3�� |
| B��25��ʱ��pH=3�Ĵ�����Һ��pH=11������������Һ�������Ϻ�pH=7 |
| C���ڴ�ˮ�м����������������泥���������ˮ�ĵ��� |
| D������ˮ�������Ϻ�������Һ�п��ܴ��ڣ�c��Cl-����c��NH4+����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com