
£Ø12·Ö£©ÓĆŹÆÓĶĮŃ»ÆŗĶĮŃ½ā¹ż³ĢµĆµ½µÄŅŅĻ©”¢±ūĻ©Ą“ŗĻ³É±ūĻ©ĖįŅŅõ„µÄĀ·ĻßČēĻĀ£ŗ

øł¾ŻŅŌÉĻ²ÄĮĻŗĶÄćĖłŃ§µÄ»ÆѧÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©±ūĻ©£ØCH3CH=CH2£©ÖŠŗ¬ÓŠµÄ¹ŁÄÜĶÅ £ØĢīĆū³Ę£©£¬AÖŠ¹ŁÄÜĶŵĵē×ÓŹ½__________ ”£
£Ø2£©ÓÉ CH2=CH2ÖʵĆÓŠ»śĪļAµÄ»Æѧ·½³ĢŹ½£ŗ _______ _£¬·“Ó¦ĄąŠĶŹĒ __”£
£Ø3£©AÓėBŗĻ³É±ūĻ©ĖįŅŅõ„µÄ»Æѧ·“Ó¦·½³ĢŹ½ŹĒ£ŗ_____________________________£¬
·“Ó¦ĄąŠĶŹĒ ”£
£Ø4£©ÓÉŹÆÓĶĮŃ½ā²śĪļŅŅĻ©ŗĻ³É¾ŪŅŅĻ©ĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ ”£
1£©CH2==CH2£«H2O CH3CH2OH
¼Ó³É·“Ó¦
CH3CH2OH
¼Ó³É·“Ó¦
£Ø2£©Ģ¼Ģ¼Ė«¼ü 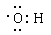
£Ø3£©CH2==CHCOOH£«CH3CH2OH CH2==CHCOOCH2CH3£«H2O Č”“ś·“Ó¦
CH2==CHCOOCH2CH3£«H2O Č”“ś·“Ó¦
£Ø4£©nCH2==CH2
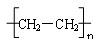
”¾½āĪö”æ£Ø1£©±ūĻ©ŹōÓŚĻ©Ģž£¬ŗ¬ÓŠĢ¼Ģ¼Ė«¼ü”£
£Ø2£©ŅŅĻ©Ņ²ŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬ŗĶĖ®·¢Éś¼Ó³É·“Ӧɜ³ÉŅŅ“¼£¬ŅŅ“¼µÄÖŠ¹ŁÄÜĶÅŹĒōĒ»ł£¬µē×ÓŹ½ĪŖ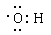 ”£
ӣ
£Ø3£©±ūĻ©Č©Ńõ»ÆÉś³É±ūĻ©ĖįB£¬½į¹¹¼ņŹ½ĪŖCH2£½CHCOOH”£±ūĻ©ĖįŗĶŅŅ“¼·¢Éśõ„»Æ·“Ӧɜ³É±ūĻ©ĖįŅŅõ„”£
£Ø4£©ŅŅĻ©Ņ²æÉŅŌ·¢Éś¼Ó¾Ū·“Ó¦£¬Éś³É¾ŪŅŅĻ©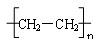 ”£
ӣ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ÅØĮņĖį |
| ”÷ |
| ÅØĮņĖį |
| ”÷ |


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø12·Ö£©ÓĆŹÆÓĶĮŃ»ÆŗĶĮŃ½ā¹ż³ĢµĆµ½µÄŅŅĻ©”¢±ūĻ©Ą“ŗĻ³É±ūĻ©ĖįŅŅõ„µÄĀ·ĻßČēĻĀ£ŗ
øł¾ŻŅŌÉĻ²ÄĮĻŗĶÄćĖłŃ§µÄ»ÆѧÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©±ūĻ©£ØCH3CH=CH2£©ÖŠŗ¬ÓŠµÄ¹ŁÄÜĶÅ £ØĢīĆū³Ę£©£¬AÖŠ¹ŁÄÜĶŵĵē×ÓŹ½__________ ”£
£Ø2£©ÓÉ CH2=CH2ÖʵĆÓŠ»śĪļAµÄ»Æѧ·½³ĢŹ½£ŗ _______ _£¬·“Ó¦ĄąŠĶŹĒ __”£
£Ø3£©AÓėBŗĻ³É±ūĻ©ĖįŅŅõ„µÄ»Æѧ·“Ó¦·½³ĢŹ½ŹĒ£ŗ_____________________________£¬
·“Ó¦ĄąŠĶŹĒ ”£
£Ø4£©ÓÉŹÆÓĶĮŃ½ā²śĪļŅŅĻ©ŗĻ³É¾ŪŅŅĻ©ĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013Ń§ÄźÉ½Ī÷“óѧø½Źō֊ѧø߶žŹī¼Ł8ŌĀæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø8·Ö£©ÓĆŹÆÓĶĮŃ»ÆŗĶĮŃ½ā¹ż³ĢµĆµ½µÄŅŅĻ©”¢±ūĻ©Ą“ŗĻ³É±ūĻ©ĖįŅŅõ„µÄĀ·ĻßČēĻĀ£ŗ
øł¾ŻŅŌÉĻ²ÄĮĻŗĶÄćĖłŃ§µÄ»ÆѧÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÓÉCH2=CH2ÖʵĆÓŠ»śĪļAµÄ»Æѧ·½³ĢŹ½£ŗ £¬·“Ó¦ĄąŠĶŹĒ ”£
£Ø2£©AÓėBŗĻ³É±ūĻ©ĖįŅŅõ„µÄ»Æѧ·“Ó¦·½³ĢŹ½ŹĒ£ŗ ”£øĆ·“Ó¦µÄĄąŠĶŹĒ ”£
£Ø3£©ÓÉŹÆÓĶĮŃ½ā²śĪļŅŅĻ©ŗĻ³É¾ŪŅŅĻ©ĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÉĀĪ÷Ź”Ī÷°²Ņ»ÖŠøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
ÓĆŹÆÓĶĮŃ»ÆŗĶĮŃ½ā¹ż³ĢµĆµ½µÄŅŅĻ©”¢±ūĻ©Ą“ŗĻ³É±ūĻ©ĖįŅŅõ„µÄĀ·ĻßČēĻĀ£ŗ
ÓŠ»śĪļA + ÓŠ»śĪļ C ”ś CH2=CHCOOCH2CH3(±ūĻ©ĖįŅŅõ„)
øł¾ŻŅŌÉĻ²ÄĮĻŗĶÄćĖłŃ§µÄ»ÆѧÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÓÉCH2=CH2ÖʵĆÓŠ»śĪļAµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ£ŗ £»
£Ø2£©±ūĻ©Č©[ CH2ØTCH CHO ]ÖŠŗ¬ÓŠµÄ¹ŁÄÜĶÅ£ŗ £ØĢīĆū³Ę£©£»
£Ø3£©AÓėCŗĻ³É±ūĻ©ĖįŅŅõ„µÄ»Æѧ·“Ó¦·½³ĢŹ½ŹĒ£ŗ
_________________________________________________________________£»
øĆ·“Ó¦µÄĄąŠĶŹĒ ·“Ó¦”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com