
�����о�����Դ��δ��Ϲ������Ȼ�ѧѭ���ֽ�ˮ�� H2����ˮ��Ӧ��ϵ�м���һ���м��������ͬ�ķ�Ӧ�Σ����ս�ˮ�ֽ�ΪH2 �� O2 ������һ�ֽ�Լ��Դ����ʡ��Ӧ���ϵļ�������ͼ���Ȼ�ѧѭ�������������̣�

(1)ʵ���ã�1 g H2 ȼ������Һ̬ˮ�ų� 142.9 kJ �����������ʾ����ȼ�յ��Ȼ�ѧ����ʽΪ_______________________________________________
______________________________________________________________��
(2)�������̲���ѭ����������________��________(�ѧʽ)�����ѽ��еķ�Ӧ��________(�����)��
(3)����Ȼ�ж�������;�㷺���ù����廯���������������ڽϵ��¶��¾������з�Ӧʹˮ�ֽ���������������
��CaBr2 �� 2H2O===Ca(OH)2 �� 2HBr����
�ڡ���
��HgBr2 �� Ca(OH)2===CaBr2 �� HgO��H2O��
��2HgO===2Hg �� O2����
��Ӧ�ڵĻ�ѧ����ʽΪ___________________________________________��
(4)�ϳɰ��õ� H2 ���Լ���Ϊԭ���Ƶá��йػ�ѧ��Ӧ�������仯����ͼ��ʾ���� CH4 (g)�� H2O(g)��Ӧ����CO(g)�� H2 (g)���Ȼ�ѧ����ʽΪ_________________________________________________________��
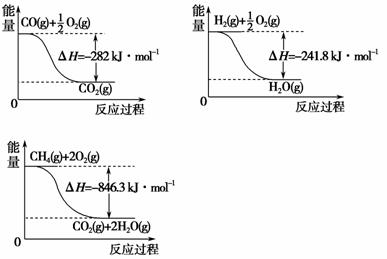
������(4)������ͼ��д����Ӧ���Ȼ�ѧ����ʽ��
CO(g)��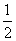 O2 (g) ===CO2 (g)
O2 (g) ===CO2 (g)
��H����282 kJ��mo��1���� ��
H2 (g) ��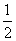 O2(g)===H2O(g)
O2(g)===H2O(g)
��H����241.8 kJ��mo��1���� ��
CH4(g)�� 2O2(g)===CO2(g)��2H2O(g)
��H����846.3 kJ��mo��1���� �ۡ�
�ɸ�˹���ɣ��á��ۣ�(�٣��ڡ�3)��������Ӧ�Ħ�H����161.1 kJ��mo��1��
�𰸡�(1)2H2 (g) ��O2 (g) ===2H2O (l) ����H����571.6 kJ��mo��1
(2)SO2�� I2 ����
(3)Hg �� 2HBr===HgBr2 �� H2��
(4)CH4(g)��H2O(g)===CO(g)��3H2(g) ��H����161.1 kJ��mol��1


 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ�Ұ�ҩ�����ڸ߷�������E���Ƴɻ��ͳ�Чҩ������˾ƥ��
(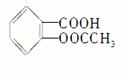 )��������ij�߷��Ӿۺ����ϣ��γɻ��ͳ�Чҩ��������һ�ֽṹ��ʽΪ��
)��������ij�߷��Ӿۺ����ϣ��γɻ��ͳ�Чҩ��������һ�ֽṹ��ʽΪ��
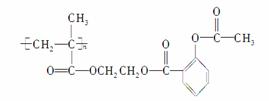
�Իش��������⣺
(1)����ṹ��ʽΪ________________________________��
(2)���ͷ�Ӧ�Ļ�ѧ����ʽΪ
________________________________________________________________________
________________________________________________________________________��
(3)��˾ƥ���ڼ���������(NaOH)����ˮ�ⷴӦ�Ļ�ѧ����ʽΪ
________________________________________________________________________
________________________________________________________________________��
(4)���ָ߷����������ɵ��巢���ۺϷ�Ӧ�õ��ģ�д������Ľṹ��ʽ��
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ���ӦΪ��N2(g)��3H2(g)2NH3(g)��ͼ1��ʾ��һ�����¶��´˷�Ӧ�����е������ı仯��ͼ2��ʾ��2 L���ܱ������з�ӦʱN2�����ʵ�����ʱ��ı仯���ߡ�ͼ3��ʾ�������������������£��ı���ʼ�����������ʵ����Դ˷�Ӧƽ���Ӱ�졣

����˵����ȷ���� (����)��
A���÷�ӦΪ�Է���Ӧ����ͼ1�ɵü����ʵ��Ĵ�����E�ͦ�H����С
B��ͼ2��0��10 min�ڸ÷�Ӧ��ƽ������v(H2)��0.045 mol��L��1��min��1����11 min�������������䣬ѹ�����������Ϊ1 L����n(N2)�ı仯����Ϊd
C��ͼ3��a��b��c����������ƽ��״̬�У���Ӧ��N2��ת������ߵ���b��
D��ͼ3��T1��T2��ʾ�¶ȣ���Ӧ�¶��µ�ƽ�ⳣ��ΪK1��K2����T1>T2��K1>K2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ס�������������ͬ�������壬�ڿ�����ȼ�յõ����������������ʱ����P4O6����������ʱ����P4O10��
(1)��֪298 Kʱ���ס�������ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�ΪP4(s������)��5O2(g)===P4O10(s) ��H1����2 983.2 kJ��mol��1
P(s������)��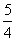 O2(g)===
O2(g)===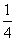 P4O10(s) ��H2����738.5 kJ��mol��1
P4O10(s) ��H2����738.5 kJ��mol��1
����¶��°���ת��Ϊ�����Ȼ�ѧ����ʽΪ_______________________
_______________________________________________________________��
(2)��֪298 Kʱ���ײ���ȫȼ�յ��Ȼ�ѧ����ʽΪP4(s������)��3O2(g)===P4O6(s)
��H����1 638 kJ��mol��1����ij�ܱ������м���62 g����50.4 L����(��״��)����������ʹ֮ǡ����ȫ��Ӧ�������õ���P4O10��P4O6�����ʵ���֮��Ϊ________����Ӧ�����зų�������Ϊ________��
(3)��֪����PCl3�ķ��ӽṹ��ͼ��ʾ�����ṩ���»�ѧ���ļ���(kJ��mol��1)��P��P 198��Cl��Cl 243��P��Cl 331����ӦP4(s������)��6Cl2(g)===4PCl3(s)�ķ�Ӧ��
��H��________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ȼ�ѧ����ʽ�У���ȷ���� (����)��
A�������ȼ����Ϊ890.3 kJ��mol��1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪCH4(g)��2O2(g)===CO2(g)��2H2O(g)����H����890.3 kJ��mol��1
B��500 �桢30 MPa�£���0.5 mol N2(g)��1.5 mol H2(g)�����ܱ������г�ַ�Ӧ����NH3(g)����19.3 kJ�����Ȼ�ѧ����ʽΪN2(g)��3H2(g)2NH3(g)����H����38.6 kJ��mol��1
C��HCl��NaOH��Ӧ���к��Ȧ�H����57.3 kJ��mol��1����H2SO4��Ca(OH)2��Ӧ���к��Ȧ�H��2��(��57.3) kJ��mol��1
D����101 kPaʱ��2 g H2��ȫȼ������Һ̬ˮ���ų�285.8 kJ ����������ȼ�յ��Ȼ�ѧ����ʽΪ2H2(g)��O2(g)===2H2O(l)����H����571.6 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ���ܸ�AB���˷ֱ���д�С��ͬ��������ȵĿ���ͭ��Ϳ��������ڸܸ˲�ʹ����ˮ�б���ƽ�⣬Ȼ��С�ĵ���ˮ���зֱ����ŨCuSO4��Һ��ŨFeSO4 ��Һ��һ��ʱ��������йظܸ˵�ƫ���ж���ȷ����(ʵ������У�����������ĸ����仯) (����)��
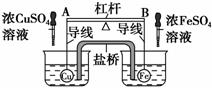
A���ܸ�Ϊ������Ե��ʱ����ΪA�˸�B�˵�
B���ܸ�Ϊ������Ե��ʱ����ΪA�˵�B�˸�
C�����ܸ�Ϊ����ʱ��A�˵�B�˸�
D�����ܸ�Ϊ����ʱ��A�˸�B�˵�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ԭ��Ӧ��2Ag��(aq)��Cu(s)===Cu2��(aq)��2Ag(s)��Ƶ�ԭ�����ͼ��ʾ��
��ش��������⣺

(1)�缫X�IJ�����________���������ҺY��___________________ _________________________________________________��
(2)���缫Ϊ��ص�________���������ĵ缫��ӦΪ__________________��
(3)���·�еĵ�������Ϊ__________________________________________��
(4)�����е�Cl����________���ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼΪ��������Ҫ�ɷ�ʾ��ͼ������˵������ȷ����
A���ؽ������ӿɵ��µ����ʱ���
B��������ķ�����
C��SO2��NxOy����������������
D������β���Ĵ����ŷ������������������Ϊ����֮һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������в����ڹ����л��߷��Ӳ��ϵ���(����)
A���߷��ӷ���Ĥ B��ҽ�ø߷���
C������߷��� D���ϳ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com