
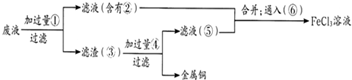
 ����һ�ֵ��͵Ľ����л������ʵ���ҳ��������Ȼ������ͻ����ϩ�ڼ��������·�Ӧ�õ�����Ӧԭ��ΪFeCl2+2C5H6+2KOH��Fe��C5H5��2+2KCl+2H2O����ï�����۵�Ϊ172��173�棬��100�濪ʼ���������������ѡ������������ܼ���������ˮ���Լ�ͷ����������ȶ���ij�о�С����Ƶ�ʵ�鷽�����Ʊ�װ��ʾ��ͼ���£�
����һ�ֵ��͵Ľ����л������ʵ���ҳ��������Ȼ������ͻ����ϩ�ڼ��������·�Ӧ�õ�����Ӧԭ��ΪFeCl2+2C5H6+2KOH��Fe��C5H5��2+2KCl+2H2O����ï�����۵�Ϊ172��173�棬��100�濪ʼ���������������ѡ������������ܼ���������ˮ���Լ�ͷ����������ȶ���ij�о�С����Ƶ�ʵ�鷽�����Ʊ�װ��ʾ��ͼ���£�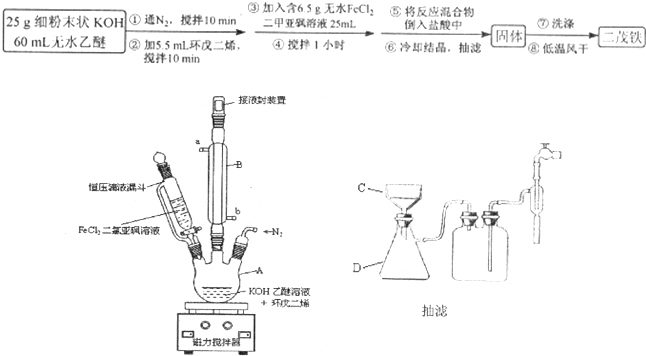



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ڢ� | B���ܢ� |
| C���ۢܢݢ� | D���٢ܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ˮ�����Һ©�������������Ȼ�̼�����ã��ɽ�����ȡ���²�Һ���� |
| B����AlCl3��Һ�еμӰ�ˮ��������ɫ�������ټ������NaHSO4��Һ��������ʧ |
| C�����������Գ�ȥ�������е�����̼�������� |
| D���ں�FeCl2���ʵ�FeCl3��Һ��ͨ����Cl2��ּ��ȣ���ȥ������Cl2����õ�������������FeCl3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ | B������ | C����ȡ | D����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CH4��C2H6 |
| B��C2H4��C3H6 |
| C��C2H2��C6H6 |
| D��C5H10��C6H6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������������ˮ��c��CH3COO-��һ������c��Na+����c��NH4+��֮�� |
| B������Һ�ɵ����ʵ���Ũ�ȡ��������NaOH��Һ��CH3COOH��Һ��϶��� |
| C����������NaOH����Һ������Ũ��Ϊc��CH3COO-����c��Na+����c��OH-����c��H+�� |
| D������Һ��pH=3��CH3COOH��pH=11��NaOH��Һ�������϶��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com