
·ÖĪö ¢ŁÓĆpHŹŌÖ½¼ģ²ā£¬ČÜŅŗ³ŹĒæĖįŠŌ£¬ĖµĆ÷ČÜŅŗÖŠ“ęŌŚH+£»ŅĄ¾ŻĄė×Ó¹²“ęæÉÖŖŅ»¶Ø²»“ęŌŚCO32-£¬AlO2-£»
¢ŚČ”ČÜŅŗŹŹĮ棬¼ÓČėÉŁĮæCCl4ŗĶŹżµĪŠĀÖĘĀČĖ®£¬Õńµ“£¬CCl4²ć³Ź×ĻŗģÉ«£¬ĖµĆ÷ČÜŅŗÖŠŅ»¶Øŗ¬ÓŠI-£¬ŅĄ¾ŻĄė×Ó¹²“ę·ÖĪöÅŠ¶ĻæÉÖŖ£¬Ņ»¶Ø²»ŗ¬ÓŠFe3+£»
¢ŪĮķČ”ČÜŅŗŹŹĮ棬֚µĪ¼ÓČėNaOHČÜŅŗ£»
a£®ČÜŅŗ“ÓĖįŠŌ±äĪŖÖŠŠŌ£¬ÖŠŗĶĖį£»
b£®ČÜŅŗÖš½„²śÉś³Įµķ£¬·ÖĪöŃōĄė×ÓÖŠÖ»ÓŠĀĮĄė×Ó³Įµķ£»
c£®³ĮµķĶźČ«Čܽā£¬Ö¤Ć÷Éś³ÉµÄ³ĮµķŹĒĒāŃõ»ÆĀĮ£¬ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠAl3+£»
d£®×īŗó¼ÓČČČÜŅŗ£¬ÓŠĘųĢå·Å³ö£¬øĆĘųĢåÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬Ö¤Ć÷Éś³ÉµÄĘųĢåŹĒ°±Ęų£¬ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠļ§øłĄė×Ó£¬ÓÉ“Ė·ÖĪö½ā“š£®
½ā“š ½ā£ŗ¢ŁÓĆpHŹŌÖ½¼ģ²ā£¬ČÜŅŗ³ŹĒæĖįŠŌ£¬ĖµĆ÷ČÜŅŗÖŠ“ęŌŚH+£»ŅĄ¾ŻĄė×Ó¹²“ęæÉÖŖŅ»¶Ø²»“ęŌŚCO32-£¬AlO2-£»
¢ŚČ”ČÜŅŗŹŹĮ棬¼ÓČėÉŁĮæCCl4ŗĶŹżµĪŠĀÖĘĀČĖ®£¬Õńµ“£¬CCl4²ć³Ź×ĻŗģÉ«£¬ĖµĆ÷ČÜŅŗÖŠŅ»¶Øŗ¬ÓŠI-£¬ŅĄ¾ŻĄė×Ó¹²“ę·ÖĪöÅŠ¶ĻæÉÖŖ£¬Ņ»¶Ø²»ŗ¬ÓŠFe3+£»
¢ŪĮķČ”ČÜŅŗŹŹĮ棬֚µĪ¼ÓČėNaOHČÜŅŗ£»
a£®ČÜŅŗ“ÓĖįŠŌ±äĪŖÖŠŠŌ£¬ÖŠŗĶĖį£»
b£®ČÜŅŗÖš½„²śÉś³Įµķ£¬·ÖĪöŃōĄė×ÓÖŠÖ»ÓŠĀĮĄė×Ó³Įµķ£»
c£®³ĮµķĶźČ«Čܽā£¬Ö¤Ć÷Éś³ÉµÄ³ĮµķŹĒĒāŃõ»ÆĀĮ£¬ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠAl3+£»
d£®×īŗó¼ÓČČČÜŅŗ£¬ÓŠĘųĢå·Å³ö£¬øĆĘųĢåÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬Ö¤Ć÷Éś³ÉµÄĘųĢåŹĒ°±Ęų£¬ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠļ§øłĄė×Ó£»
£Ø1£©ÓÉ¢ŁÓĆpHŹŌÖ½¼ģ²ā£¬ČÜŅŗ³ŹĒæĖįŠŌ£¬ĖµĆ÷ČÜŅŗÖŠ“ęŌŚH+£»æÉŅŌÅųżCO32-£¬AlO2-µÄ“ęŌŚ£¬¹Ź“šĪŖ£ŗH+£»CO32-£¬AlO2-£»
£Ø2£©ÓÉ¢ŚæÉŅŌÖ¤Ć÷I-Ņ»ŃłµÄ“ęŌŚ£¬CCl4²ć³öĻÖµāµ„ÖŹµÄŃÕÉ«Ö¤Ć÷ŗ¬I-£¬Fe3+ŌŚøĆ»·¾³ÖŠÓėI-²»Äܹ²“ę£¬ŅĄ¾ŻĄė×Ó¹²“ęĶ¬Ź±ÅųżFe3+µÄ“ęŌŚ£¬
¹Ź“š°øĪŖ£ŗI-£»Fe3+£»
£Ø3£©¢ŪÖ¤Ć÷ČÜŅŗÖŠŅ»¶Øŗ¬ÓŠAl3+£¬NH4+£»Ć»ÓŠMg2+µÄ“ęŌŚ£¬¹Ź“š°øĪŖ£ŗAl3+£¬NH4+£»Mg2+£®
µćĘĄ ±¾Ģāæ¼²éĮĖĄė×Ó¼ģŃéµÄŹµŃé·½·ØŗĶ·“Ó¦ĻÖĻóµÄÅŠ¶Ļ£¬Ąė×ÓŠŌÖŹŗĶĄė×Ó·“Ó¦½ųŠŠµÄĄė×Ó¹²“ę·ÖĪöŹĒ½āĢā¹Ų¼ü£¬ĢāÄæÄѶČÖŠµČ£®


 Ó„ÅɽĢøØĻĪ½Ó½Ģ²ÄŗÓ±±½ĢÓż³ö°ęÉēĻµĮŠ“š°ø
Ó„ÅɽĢøØĻĪ½Ó½Ģ²ÄŗÓ±±½ĢÓż³ö°ęÉēĻµĮŠ“š°ø ³õÖŠŹīĘŚĻĪ½ÓĻµĮŠ“š°ø
³õÖŠŹīĘŚĻĪ½ÓĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2 | B£® | 3 | C£® | 4 | D£® | 5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NH4ClµÄµē×ÓŹ½£ŗ | |
| B£® | µŖĘųµÄ½į¹¹Ź½£ŗ£ŗN”ŌN£ŗ | |
| C£® | ĀČ»ÆĒā·Ö×ӵĊĪ³É¹ż³ĢæÉÓƵē×ÓŹ½±ķŹ¾Ź½£ŗ | |
| D£® | ÖŲĖ®µÄ»ÆѧŹ½ĪŖ 21H2O£Ø»ņD2O£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 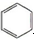 +HNO3$”ś_{”÷}^{ÅØĮņĖį}$ +HNO3$”ś_{”÷}^{ÅØĮņĖį}$ 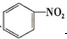 +H2O£»Č”“ś·“Ó¦ +H2O£»Č”“ś·“Ó¦ | |
| B£® | CH2ØTCH2+Br2”śCH2Br-CH2Br£»¼Ó³É·“Ó¦ | |
| C£® | 2CH3CH2OH+O2$”ś_{”÷}^{Cu}$2CH3CHO+2H2O£»Č”“ś·“Ó¦ | |
| D£® | CH3COOH+CH3CH2OH$?_{”÷}^{ÅØĮņĖį}$CH3COOCH2CH3+H2O£»õ„»Æ·“Ó¦Ņ²ŹōÓŚČ”“ś·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ėłŗ¬·Ö×ÓŹż²»ĻąµČ | B£® | Ėłŗ¬Ō×ÓŹż×ÜŹżĻąµČ | ||
| C£® | Ėłŗ¬µē×ÓŹżĻąµČ | D£® | Ėłŗ¬µŖŌ×ÓŹżĻąµČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŹÆÓĶµÄ“ß»ÆÖŲÕūŗĶĆŗµÄøÉĮó¾łæÉŅŌµĆµ½·¼ĻćĢž£¬ĖµĆ÷ŹÆÓĶŗĶĆŗÖŠŗ¬ÓŠ·¼ĻćĢž | |
| B£® | ŹÆÓĶĮŃ½āµÄÄæµÄÖ÷ŅŖŹĒĪŖĮĖµĆµ½øü¶ąµÄĘūÓĶ | |
| C£® | ŹÆÓĶ·ÖĮóµĆµ½µÄ²śĪļæÉÓĆĄ“ŻĶČ”äåĖ®ÖŠµÄäå | |
| D£® | ŹÆÓĶĮŃ»ÆÖ÷ŅŖµĆµ½µÄŹĒŅŅĻ©”¢±ūĻ©µČĘųĢ¬Ģž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
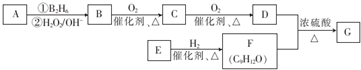
 £»øĆ·“Ó¦µÄ·“Ó¦ĄąŠĶĪŖõ„»Æ·“Ó¦»ņČ”“ś·“Ó¦£®
£»øĆ·“Ó¦µÄ·“Ó¦ĄąŠĶĪŖõ„»Æ·“Ó¦»ņČ”“ś·“Ó¦£®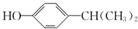 £®
£® ĪŖŌĮĻŅ²æÉŗĻ³ÉF£¬Ēė²Īæ¼ĢāÄæÖŠµÄĻą¹ŲŠÅĻ¢Š“³öĻąÓ¦µÄŗĻ³ÉĀ·ĻßĶ¼£Ø·“Ó¦Ģõ¼žÖŠµÄŹŌ¼ĮŠ“ŌŚ¼żĶ·ÉĻ·½£¬ĘäĖūŠ“ŌŚ¼żĶ·ĻĀ·½£©£ŗ
ĪŖŌĮĻŅ²æÉŗĻ³ÉF£¬Ēė²Īæ¼ĢāÄæÖŠµÄĻą¹ŲŠÅĻ¢Š“³öĻąÓ¦µÄŗĻ³ÉĀ·ĻßĶ¼£Ø·“Ó¦Ģõ¼žÖŠµÄŹŌ¼ĮŠ“ŌŚ¼żĶ·ÉĻ·½£¬ĘäĖūŠ“ŌŚ¼żĶ·ĻĀ·½£©£ŗ £®
£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com