
���� ��1������1mol������̼�������������ɼ״���ˮ���ȣ�����Ȼ�ѧ����ʽ��д������ע���ʾۼ�״̬�ͷ�Ӧ�ʱ�д����
��2������̼Ԫ���غ����ϳɼ״����ʵ����������Ȼ�ѧ����ʽ����ų���������
��3�����ݺϳɼ״����Ȼ�ѧ����ʽ��H2O��g���TH2O��l����H=-44kJ/mol�����ø�˹���ɼ���õ�1mol������̼��Ӧ�ų��������õ�22g������̼��0.5mol������̼��Ӧ�ų���������
��� �⣺��1����֪4.4g CO2������H2������������CH3OH�����ˮ����ʱ�ų�4.95kJ����������1mol������̼ȫ����Ӧ����49.5KJ������Ȼ�ѧ����ʽ��д����д���Ȼ�ѧ����ʽΪ��CO2��g��+3H2��g��=CH3OH��g��+H2O��g����H=-49.5KJ/mol��
�ʴ�Ϊ��CO2��g��+3H2��g���TCH3OH��g��+H2O��g����H=-49.5 kJ/mol��
��2��n��CH3OH��=n��CO2��=$\frac{4.48��103L}{22.4L/mol}$��22%=44mol��ÿ����1mol CH3OH��g������49.5kJ����˹����зų�����Ϊ����44��49.5��kJ=2178kJ��
�ʴ�Ϊ��44mol��2178��
��3����H2O��g���TH2O��l����H=-44kJ/mol�Լ���1���е��Ȼ�ѧ����ʽ��֪��4.48m3�����ۺ�Ϊ��״������CO2���ʵ���Ϊ1mol��1mol CO2��ȫ�ϳ�CH3OH������Һ̬ˮʱ�ų�����Ϊ44kJ+49.5kJ=93.5kJ����22g CO2��0.5mol CO2��H2��Ӧʱ���ų�����Ϊ93.5kJ��2=46.75kJ��
�ʴ�Ϊ��46.75kJ��
���� ���⿼�����Ȼ�ѧ����ʽ��д�ͼ���Ӧ�ã����ո�˹���ɼ����ǹؼ�����Ŀ�Ѷ��еȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 40.5% | B�� | 60.6% | C�� | 81.0% | D�� | 100% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
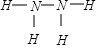 ����֪Һ̬�µı�ȼ����Ϊ-622kJ/mol��д������ȼ�շ������Ȼ�ѧ����ʽ��N2H4��l��+O2��g��=N2��g��+2H2O��l������H=-622KJ/mol��
����֪Һ̬�µı�ȼ����Ϊ-622kJ/mol��д������ȼ�շ������Ȼ�ѧ����ʽ��N2H4��l��+O2��g��=N2��g��+2H2O��l������H=-622KJ/mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 5min����O2��ʾ�ķ�Ӧ����Ϊ0.12mol/��L•min�� | |
| B�� | �����������ʹ�÷�Ӧ�Ļ�ѧ��Ӧ���ʼӿ죬��H��С | |
| C�� | SO2��ƽ��Ũ��Ϊ0.12mol/L | |
| D�� | �ﵽƽ��ʱ���������������������÷�Ӧ�Ļ�ѧ��Ӧ���ʼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
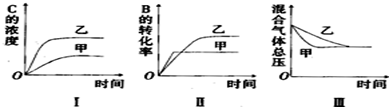
| A�� | ͼI�о��Ŀ����Dz�ͬ�����Է�Ӧ��Ӱ�죬����ʹ�õĴ���Ч�ʽϸ� | |
| B�� | ͼ���о��Ŀ�����ѹǿ�Է�Ӧ��Ӱ�죬�Ҽ�ѹǿ�ϸ� | |
| C�� | ͼ���о��Ŀ������¶ȶԷ�Ӧ��Ӱ�죬�Ҽ��¶Ƚϸ� | |
| D�� | ͼ���о��Ŀ����Dz�ͬ�����Է�Ӧ��Ӱ�죬����ʹ�õĴ���Ч�ʽϸ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com