
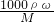 �����жϣ�
�����жϣ�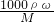 ��֪����=
��֪����=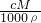 =
=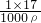 =1.7%
=1.7% �����ڰ�ˮ�ܶȦѣ�1�����Ԧ�=1.7%
�����ڰ�ˮ�ܶȦѣ�1�����Ԧ�=1.7% ��1.7%����B����
��1.7%����B����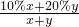 =10%+10%
=10%+10%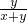 =10%+10%
=10%+10%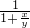 ����ˮŨ��Խ���ܶ�ԽС������x��y����
����ˮŨ��Խ���ܶ�ԽС������x��y����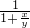 ��
�� ������10%+10%
������10%+10%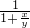 ��15%����D����
��15%����D����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧ�� | C-H | C-F | H-F | F-F |
| ���� | 414 | 489 | 565 | 158 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧ�� | C-H | C-F | H-F | F-F |
| ���� | 414 | 489 | 565 | 158 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
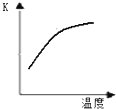 ��ѧ��һֱ�����ڡ��˹��̵������·����о���
��ѧ��һֱ�����ڡ��˹��̵������·����о���| 4 |
| 27 |
| 4 |
| 27 |
| c(NH3?H2O) |
| c(OH-) |
| c(NH4+)?c(OH-) |
| c(NH3?H2O) |
| c(H+) |
| c(OH-) |
| ʱ��/���ʵ��� | n��NH3�� ��mol�� | n��O2 �� ��mol�� | n��NO�� ��mol�� |
| ��ʼ | 1.600 | 3.200 | 0.000 |
| ��2min | a | 2.700 | 0.4000 |
| ��4min | 0.600 | 1.950 | 1.000 |
| ��6min | 0.600 | 1.950 | 1.000 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��ѧ�� | C-H | C-F | H-F | F-F |
| ���� | 414 | 489 | 565 | 158 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ����ѧ���и߶����ϣ����л�ѧ�Ծ������ƣ��������棩 ���ͣ������
| ��ѧ�� | C-H | C-F | H-F | F-F |
| ���� | 414 | 489 | 565 | 158 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com