
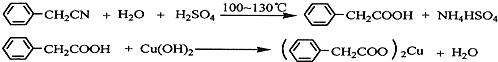
���� ��1��ϡ��Ũ����ʱ�����ܶȴ����Һ���뵽�ܶ�С����Һ�У�
��2��c�����������ܣ��������л������ã�
��3��������������ˮ��
�����������ˮ�����Բ��ù��˷������룻
��4��������������ˮ����ˮ�е��ܽ�Ƚ�С���ɷ�Ӧ ��֪��40g���������ɱ�����Ϊ40g��$\frac{136}{117}$=46.5g��
��֪��40g���������ɱ�����Ϊ40g��$\frac{136}{117}$=46.5g��
��5���������û��ϴ�Ӹɾ��������������������ӣ��������������ữ�����ᱵ���飻
��6������������ˮ�������Ҵ���
��� �⣺��1��ϡ��Ũ����ʱ�����ܶȴ����Һ���뵽�ܶ�С����Һ�У�����Ũ�ȴ���ˮ������ϡ��Ũ����ʱӦ�ý�Ũ�������ձ��ڱڵ���ˮ�в����Ͻ��裬������Һ�μ�˳�����ȼ�ˮ���ټ���Ũ���ᣬ
�ʴ�Ϊ���ȼ�ˮ���ټ���Ũ���
��2��c�����������ܣ��������л������ã�������÷�Ӧ���߲�����ʣ�
�ʴ�Ϊ�����������ܣ���������ʹ�����ķ�ӦҺ��������
��3��������������ˮ�����Լ�����ˮ���ڱ�����������
�����������ˮ�����Բ��ù��˷������룬��Ҫ���������ձ���©������������
�ʴ�Ϊ�����ڱ�����������BCE��
��4��������������ˮ����ˮ�е��ܽ�Ƚ�С�������ؽᾧ�ķ��������ᴿ��������������ˮ����ˮ�е��ܽ�Ƚ�С�����ᴿ������ķ������ؽᾧ���ɷ�Ӧ ��֪��40g���������ɱ�����Ϊ40g��$\frac{136}{117}$=46.5g�����յõ�44g��Ʒ��������IJ����ǣ�$\frac{44g}{46.5g}$��100%=95%��
��֪��40g���������ɱ�����Ϊ40g��$\frac{136}{117}$=46.5g�����յõ�44g��Ʒ��������IJ����ǣ�$\frac{44g}{46.5g}$��100%=95%��
�ʴ�Ϊ���ؽᾧ��95%��
��5���������û��ϴ�Ӹɾ��������������������ӣ��������������ữ�����ᱵ���飬����鷽��Ϊȡ����ϴ��Һ������ϡ���ᣬ�ټ�AgNO3��Һ���ް�ɫ���dz��֣�˵��ϴ�Ӹɾ���
�ʴ�Ϊ��ȡ����ϴ��Һ������ϡ���ᣬ�ټ�AgNO3��Һ���ް�ɫ���dz��֣�
��6������������ˮ�������Ҵ������Ҵ��ܽⱽ����������������ܽ�ȣ����ڳ�ַ�Ӧ��
�ʴ�Ϊ�����������ܽ�ȣ����ڳ�ַ�Ӧ��
���� ���⿼���Ʊ�ʵ�鷽����ƣ�Ϊ��Ƶ���㣬���պϳɷ�Ӧ��ʵ��װ�õ�����Ϊ���Ĺؼ����ۺϿ���ѧ��ʵ�鼼�ܺͷ������������������Ŀ�Ѷ��еȣ�


 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��īˮ�ӵ���ˮʹ����ˮ��� | B�� | �����������ڻ���ˮ | ||
| C�� | ˮ���ߴ��� | D�� | �����ڳ�ʪ�Ŀ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | ������ƽ | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� |  |  |  |  |  | |
| ��� | a | b | c | d | e | f |
| ʵ�� ���� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij��ѧʵ��С����0.2000 mol/L������KMnO4��Һ�ⶨ���ᾧ��Ĵ��ȣ����ᾧ�廯ѧʽΪH2C2O4•2H2O�����ʲ���KMnO4��Ӧ����ʵ�鲽�����£�
ij��ѧʵ��С����0.2000 mol/L������KMnO4��Һ�ⶨ���ᾧ��Ĵ��ȣ����ᾧ�廯ѧʽΪH2C2O4•2H2O�����ʲ���KMnO4��Ӧ����ʵ�鲽�����£�| �ζ����� | ������Һ��� | ����KMnO4��Һ��� | |
| �ζ�ǰ����/m L | �ζ������/m L | ||
| ��һ�� | 25.00 | 0.20 | 20.58 |
| �ڶ��� | 25.00 | 4.00 | 24.40 |
| ������ | 25.00 | 2.38 | a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������̼�IJ�����������Դ�����һ����Ҫս�Է���
������̼�IJ�����������Դ�����һ����Ҫս�Է���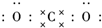 ��������ѧ���������ǹ��ۼ���
��������ѧ���������ǹ��ۼ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����ʳ�����������/mL | 0.02 | 0.03 | 0.00 |
| ����ʳ������ն���/mL | 25.01 | 25.04 | 25.02 |
| �������Ʊ�Һ�����������/mL | 0.01 | 0.03 | 0.04 |
| �������Ʊ�Һ������ն���/mL | 12.52 | 12.55 | 12.58 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 mol�����к���1.204��1024����ԭ�ӣ��ڱ�״����ռ�����22.4 L | |
| B�� | 1 mol������1.5 mol����������ͬ����ԭ���� | |
| C�� | ��He��H2��O2����������¶Ⱥ��ܶȶ���ͬʱ�����ǵ�ѹǿ��С��p��O2����p��He����p��H2�� | |
| D�� | �����ʵ����ĸɱ��������ǣ�����ʽΪC6H12O6��������̼ԭ����֮��Ϊ1��6����ԭ����֮��Ϊ1��3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com