
| 1000�Ѧ� |
| M |
| n |
| V |
| 1000�Ѧ� |
| M |
| 1000��1.84g/mL��98% |
| 98g/mol |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1mol�״��к���C-H����ĿΪ4NA |
| B�������£�PH=13��NaOH��Һ�к���OH-����ĿΪ0.1NA |
| C����״���£�2.24L���麬�еķ�����ĿΪ0.1NA |
| D�����³�ѹ�£�46gNO2��N2O4�Ļ�������к��е�ԭ����Ϊ3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Ȼ�þ��Һ��Mg2++2Cl-�TMg+Cl2�� |
| B��̼��þ����Һ�мӴ��CO32-+2CH3COOH�T2CH3COO-+CO2��+H2O |
| C������K2Cr2O7��Һ������˫��ˮH2O2��Cr2O72-+8H++5H2O2=2 Cr3++4O2��+9H2O |
| D����ˮ�ܽ��Ȼ���������ԭ����AgCl+2 NH3?H2O=[Ag��NH3��2]++Cl-+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
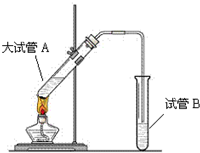 �����dz�����㡱������Ϊ�Ҵ����������������������������Ҵ���Ӧ��������ζ�����ʣ�ʵ���ҿ���ģ���һ���̣�
�����dz�����㡱������Ϊ�Ҵ����������������������������Ҵ���Ӧ��������ζ�����ʣ�ʵ���ҿ���ģ���һ���̣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
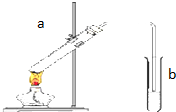 ����ͼ��ʾ��װ����ȡ�����������ش��������⣺
����ͼ��ʾ��װ����ȡ�����������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | ʵ����� | Ԥ��ʵ��Ŀ�Ļ���� |
| A | �����£���pH��ֽ�ⶨŨ��Ϊ0.1mol?L-1 Na2SiO3��Һ��Na2CO3��Һ��pH | �Ƚ�H2SiO3��H2CO3������ǿ�� |
| B | ij��Һ�м��������ữ���Ȼ�����Һ���а�ɫ�������� | ����Һ�к���SO42- |
| C | ��ij��Һ�м���2��KSCN��Һ����Һ���Ժ�ɫ��������Һ�м��뼸�����Ƶ���ˮ����Һ��Ϊ��ɫ | ����Һ��һ������Fe2+ |
| D | ��ij����ͨ����ۺ�KI�Ļ����Һ����Һ����ɫ | ������һ����Cl2 |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��BF3 |
| B��SiH4 |
| C��SF6 |
| D��PCl3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com