
����Ŀ��A��B��C��D�����ֶ�����Ԫ�أ�E�ǹ���Ԫ�ء�A��B��Cͬ���ڣ�C��Dͬ���壬A��ԭ�ӽṹʾ��ͼΪ ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C�������������δ�ɶԵ��ӣ�E����Χ�����Ų�ʽΪ3d64s2���Իش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C�������������δ�ɶԵ��ӣ�E����Χ�����Ų�ʽΪ3d64s2���Իش��������⣺
(1)д������Ԫ�صķ��ţ�A__________��B__________��C__________��D__________��
(2)�û�ѧʽ��ʾ��������Ԫ��������������Ӧˮ����������ǿ����________��������ǿ����__________��
(3)��Ԫ�ط��ű�ʾD�������ڵ�һ����������Ԫ����________���縺������Ԫ����__________��
(4)EԪ��ԭ�ӵĺ˵������__________��EԪ�������ڱ��ĵ�________���ڵ�________�壬��֪Ԫ�����ڱ��ɰ������Ų���Ϊs����p���ȣ���EԪ����________����
(5)д��DԪ��ԭ�ӹ��ɵ��ʵĵ���ʽ__________���÷�������____���Ҽ���____���м���
���𰸡�Si Na P N HNO3 NaOH Ne F 26 �� �� d ![]() 1 2
1 2
��������
A��B��C��D�����ֶ�����Ԫ�أ���A��ԭ�ӽṹʾ��ͼ��֪��x=2��A��ԭ������Ϊ14����AΪSi��A��B��Cͬ���ڣ�B��ͬ���ڵ�һ��������С��Ԫ�أ���BΪNa��C�������������δ�ɶԵ��ӣ���Cԭ�ӵ�3p�ܼ���3�����ӣ���CΪP��C��Dͬ���壬��DΪN��E�ǹ���Ԫ�أ�E����Χ�����Ų�ʽΪ3d64s2����EΪFe��
(1) ������������֪��AΪSi��BΪNa��CΪP��DΪN��
(2) �ǽ���Խǿ����������Ӧˮ��������Խǿ���ǽ�����N��P��Si��������ǿ����HNO3��������Խǿ����������Ӧˮ�������Խǿ��������Na��ǿ��������ǿ����NaOH��
(3) ͬ����Ԫ�أ�ϡ������Ԫ�صĵ�һ������������Ե�һ����������Ԫ����Ne������������ң��縺�����ʵ縺������Ԫ����F��
(4) EΪFeԪ�أ�E�ĺ�������Ų�ʽΪ1s22s22p63s23p63d64s2���ʺ˵������26��Fe�����ڱ��д��ڵ������ڵڢ��壬�����ڱ��д���d����
(5) DΪ��Ԫ�أ�ԭ�Ӻ�������Ų�ʽΪ1s22s22p3���������3��δ�ɶԵ��ӣ��ʵ����ĵ���ʽΪ��![]() ���÷�������1���Ҽ���2���м���
���÷�������1���Ҽ���2���м���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����a��b��c��d�ĸ������缫���йصķ�Ӧװ�ü����ַ�Ӧ�������£�
|
|
|
|
a��������С��b���������� | b�������������c���ޱ仯 | d���ܽ⣬c����������� | ������a������d�� |
�ɴ˿��ж������ֽ����Ļ��˳����
A. d>a>b>cB. b>c>d>aC. a>b>c>dD. a>b>d>c
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ����ȡ����Ӧ����
A. ��ϩ�ڿ�����ȼ�� B. �����������������£�����������Ӧ
C. ��FeBr3�������£�����Һ�巴Ӧ D. �Ҵ���ͭ������������������������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ����գ�
��1�����������
![]() ________________________________��
________________________________��
![]() ___________________________________��
___________________________________��
��2����������д�����ʣ�
3��4-����-4-�һ����� ______________��3��4��4-����-1-��Ȳ ______________��
��3����֪�л��オ��Ƭ��ϩ�ķ��ӽṹ�ɱ�ʾΪ��
�ٽ���Ƭ��ϩ�ķ���ʽΪ ______________���ڽ���Ƭ��ϩ���� __________��
a������ b���������� c������ d��ϩ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D���Ƕ�����Ԫ�أ�ԭ�Ӱ뾶��D>C>A>B����֪��A��Bͬ���ڣ�A��C����ͬһ���壻Cԭ�Ӻ��ڵ�����������A��Bԭ�Ӻ��ڵ�������֮�ͣ�Cԭ��������������Dԭ��������������3�����Իش�
(1)д��Ԫ�ص����ƣ�A__________��
(2)д����B��D��ɵ����ֻ�����ĵ���ʽ�ֱ�Ϊ��___________��__________��
(3)A��C������������Ӧˮ���������Խ�ǿ����__________(дˮ����ķ���ʽ)��
(4)д��C����������D������������Ӧˮ���ﷴӦ�����ӷ���ʽ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������������������Ҫװ����ͼ��A��ʾ����Ҫ�������a�Թ��а�2��3��2�����������Ũ���ᡢ�Ҵ�������Ļ����ڰ�Aͼ����װ�ã�ʹ����������������ͨ��b�Թ���ʢ�ı���̼������Һ�����뼸�η�̪��Һ���У���С�����a�Թ��еĻ��Һ���ܵ�b�Թ����ռ���Լ2mL����ʱֹͣ���ȣ�����b�Թܲ�������Ȼ���ô�����Һ��ֲ㣻�ݷ��������������������
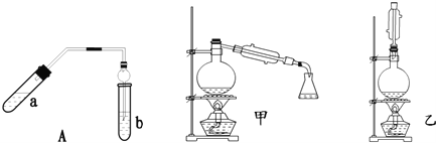
��ش��������⣺
��1��������У�������һ�����Ļ��Һ����ȷ������__��
��2����ʵ�����ú�18O���Ҵ����������ã��÷�Ӧ�û�ѧ����ʽ�ǣ�__��
��3��������У�Ҫ��С����ȣ���ԭ����__��
��4��������пɹ۲쵽b�Թ�����ϸС������ð����д���÷�Ӧ�����ӷ���ʽ��__��
��5��Aװ����ʹ�����ιܳ������������⣬��һ��Ҫ������__��������з���������������ʹ�õ�һ��������__��
��6��Ϊ������÷�Ӧ��ס�����λͬѧ�ֱ������ͼ�мס�������װ�ã���ͬѧ����Ӧ�����ȴ�����ñ���̼������Һ��ȡ��ƿ�еIJ��������Ϊ��������__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ǻϳ�ũҩ����Ҫ�м��壬��ϳ�·�����£�
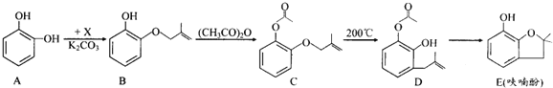
��1��A�ڿ����о��û�����ɫת��Ϊ�غ�ɫ����ԭ����____________��A�ں˴Ź�����������___________��塣
��2��B��C�ķ�Ӧ������_____________________��
��3����֪X�ķ���ʽΪC4H7Cl��д��A��B�Ļ�ѧ����ʽ��___________________��
��4��Ҫ������C��D�����˵��Լ���__________________________��
��5��B��ͬ���칹��ܶ࣬����������������______�֣�д�������ܷ���������Ӧ��ͬ���칹��Ľṹ��ʽ��__________(��дһ��)��
�ٱ������������������Ϊ��λ��ȡ���� �ۺ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�H3AsO3��H3AsO4ˮ��Һ�к���ĸ����ֵķֲ�������ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ�������pH�Ĺ�ϵ�ֱ���ͼ-1��ͼ-2��ʾ��
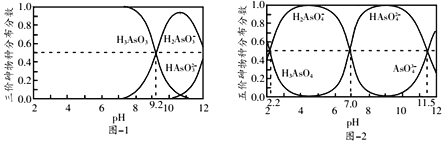
����˵��������ǣ� ��
A. H3AsO3��Na2HAsO4ˮ��Һ���Է������ֽⷴӦ
B. H3AsO4ˮ��Һ�д���:![]()
C. �� H3AsO4����Һ�м���һ������NaOH��Һ��pH=5ʱ��![]()
D. ��������0.2mol H3AsO4����Һ�м���12gNaOH���壬������Һ��pH=7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪1~20��Ԫ����A��B��C��D����Ԫ�ص�ԭ���У�������ΪA<B<C<D��AԪ�ص�ԭ�������������Ǵ�����������2����BԪ�ص�ԭ�Ӻ���M���������L���������һ�룻CԪ�ص�ԭ�Ӵ�����������������������1����DԪ�ص�ԭ�Ӻ���K�㡣L�������֮�͵���M��N�������֮�͡����ƶϣ�
��1��Ԫ��B��ԭ�ӽṹʾ��ͼ_______��
��2��C��D�γɵĻ�����ĵ���ʽ______��
��3����ҵ����AԪ�صĵ�����ȡBԪ�صĵ��ʵĻ�ѧ����ʽΪ____
��4����û����������ΪA<B<C<D�������ƣ�C��������____Ԫ�ء�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com