
 =1.5mol��
=1.5mol��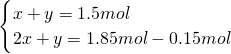


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���֪������������ճ������м�Ϊ�������ᣬ��һ��������,CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOHCH3COO-+H+ ��H>0��
��1�����³�ѹ�£��� pH =5��ϡ������Һ�У�c(CH3COO-)=_________(�����ֱ���ʽ)��
���з�����,����ʹ0��10 mol��L-1CH3COOH�ĵ���̶��������____������ĸ����
a����������0��10 mol��L-1��ϡ���� b������
c����ˮϡ����0��010 mol��L-1 d����������������
e�����������Ȼ��ƹ��� f����������0��10 mol��L-1��NaOH��Һ
��2������������пͶ��������pH������3�Ĵ����������Һ�У�������ַ�Ӧ����ֻ��һ����Һ����п��ʣ�࣬�����������������V����______V���� ,���������������������ͬ������Ӧ���������Ϊ��������_______��������
��3�������£������ΪVa mL��pH=3�Ĵ�����Һ�еμ�pH=11��NaOH��ҺVb mL����Һǡ�ó����ԣ���Va��Vb�Ĺ�ϵ�ǣ�_____________����Һ�и����ӵ�Ũ�Ȱ����ɴ�С����Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�����ص���ѧ��������ѧ�ȣ�������ѧ������������ѧ�Ծ����������� ���ͣ�������
(11��) ��֪������������ճ������м�Ϊ�������ᣬ��һ��������,CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH CH3COO��+H+ ��H��0��
CH3COO��+H+ ��H��0��
(1)�����£��� pH =5��ϡ������Һ�У�c(CH3COO��)��____________(��ʽ�����ػ���)�����з����У�����ʹ0.10 mol��L��1 CH3COOH�ĵ���̶��������______?
a����������0.10 mol��L��1��ϡ���� b������CH3COOH��Һ
c����ˮϡ����0.010 mol��L��1 d����������������
e�����������Ȼ��ƹ��� f����������0.10 mol��L��1��NaOH��Һ
(2)����������пͶ��������pH������3�Ĵ����������Һ�У�������ַ�Ӧ����ֻ��һ����Һ����п��ʣ�࣬�����������������V(����)_________V(����)����Ӧ���������Ϊ��v(����)_________v(����)��(��д������������������)
(3)�����£������ΪVa mL��pHΪ3�Ĵ�����Һ�еμ�pH=11��NaOH��ҺVb mL����Һǡ�ó����ԣ���Va��Vb�Ĺ�ϵ�ǣ�__________________��
(4)�����£���0.1 mol/L�����0.1 mol/L��������Һ��ϣ�������ҺΪ���ԣ�������Һ�и����ӵ�Ũ�Ȱ��ɴ�С����Ϊ_______________________________��
(5)��֪��90��ʱ��ˮ�����ӻ�����ΪKw = 3.8��10��13���ڴ��¶��£���pH=3�������pH = 11������������Һ�������ϣ�������Һ�е�c(H+)=______________(������λ��Ч����)mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ�����и߶��Ͻ�ѧ������⻯ѧ�Ծ��������棩 ���ͣ������
������������ճ������м�Ϊ�������ᣬ��һ��������CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH CH3COO��+H+ ��H��0
CH3COO��+H+ ��H��0
��1�������£�pH��5������Һ�У�c(CH3COO��)��______mol/L(��ȷֵ��Ҫ����ʽ���ػ���)��
��2�����з����п���ʹ0.10 mol��L-1 CH3COOH�ĵ���̶��������
a����������0.10 mol��L��1ϡ���� b������CH3COOH��Һ c����ˮϡ����0.010 mol��L��1
d���������������� e�����������Ȼ��ƹ��� f����������0.10 mol��L��1 NaOH��Һ
��3������������пͶ��������pH������3�Ĵ����������Һ�У�������ַ�Ӧ����ֻ��һ����Һ����п��ʣ�࣬�����������������V(����)_________V(����)������ڡ�����С�ڡ����ڡ���
��4����NaOH��Һ�ֱ�ζ�20.00mL0.1mol/L�����20.00mL0.1mol/L������Һ���õ���ͼ��ʾ�����ζ����ߣ���NaOH��Һ�ζ�������Һ�������� ���ͼ1����ͼ2����

��5�������£���0.1 mol/L�����0.1 mol/L��������Һ��ϣ�������ҺΪ���ԣ�������Һ�и����ӵ�Ũ�Ȱ��ɴ�С����Ϊ_______________________________��
��6����ͼ��ʾ��Һ��c(H��)��c(OH��)�Ĺ�ϵ

��M�����ڣ���Ӱ���֣������c(H��)______c(OH��)������ڡ�����С�ڡ����ڡ���
����T2�¶��£���pH��9 NaOH��Һ��pH��4 HCl��Һ��ϣ������û����Һ��pH��7����NaOH��Һ��HCl��Һ�������Ϊ ������Ϻ���Һ����ı仯���Բ��ƣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com