

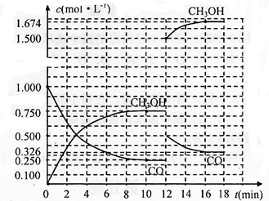
| ��Ӧʱ��/min ���ʵ���Ũ��/mol?L-1 | 0 | 3 | 10 | 12 |
| C��H2�� | 2 | |||
| C��CO�� | 0.500 | 0.250 | 0.250 | |
| C��CH3OH�� | 0 | 0.500 | 0.750 | 0.750 |

| 2��0.750mol/L |
| 10min |
| c(CH3OH) |
| c(CO)?c2(H2) |
| 0.75 |
| 0.25��0��52 |
| 0.25+0.5+0.75 |
| 1+2 |
| 1 |
| 2 |
| 1 |
| 2 |
 ��
�� ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 | ������ | ������ |
| U��+���� | Ӳ�ʲ�����+���� | |
| ��Ӧǰ����/g | a | c |
| ��ȫ��Ӧ������/g | b | d |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��һ�εζ� | �ڶ��εζ� | �����εζ� | |
| ������Һ�����mL�� | 25.00 | 25.00 | 25.00 |
| ����Һ�����mL�� | 9.99 | 10.01 | 10.60 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Na2CO3��Һ�У�c��OH-��=c��HCO3-��+2c��H2CO3��+c��H+�� |
| B��NaHCO3��Һ�У�c��Na+��=c��HCO3-��+c��CO32-��+c��H2CO3�� |
| C��pH=2��������Һ��pH=12�İ�ˮ���������Һ�У�cc��H+��+��NH4+��=c��OH-��+c��Cl-�� |
| D�������ʵ�����CH3COOH��CH3COONa��Һ��������������Һ�У�c��CH3COO-��+c��OH-��=c��H+��+c��CH3COOH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Ӧ��Ĺ���������Cu������Ϊ12.8 g |
| B����Ӧ��Ĺ��������л�����̼ |
| C����Ӧ��Ĺ�������������Ϊ13.6 g |
| D����Ӧ��Ĺ�������������������ʵ���Ϊ0.05mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
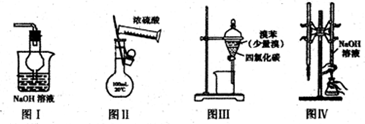
| A����ͼ����ʾװ������β���еĶ�����̼ |
| B����ͼ����ʾװ������100mL0.500mol?L-1ϡ���� |
| C����ͼ����ʾװ�ó�ȥ�屽���������� |
| D����ͼ����ʾװ�ý�����֪Ũ�ȵ�����������Һ�ⶨ����Ũ�ȵ�ʵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
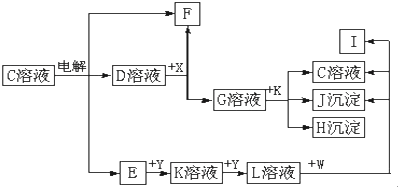
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ����ǽ�����ת��Ϊ��ѧ�ܵ�װ�� |
| B�����������������������ֱ���ȫȼ�գ����߷ų��������� |
| C�������������Һ��������������������������ʵ���֮��Ϊ1��2 |
| D����ʯȼ�Ϻ�ֲ��ȼ��ȼ��ʱ�ų�����������Դ��̫���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com