
ЎҫМвДҝЎҝБтЛбКЗј«ЖдЦШТӘөД»Ҝ№ӨФӯБПЈ¬ФЪ№ӨТөЎўЕ©ТөЎўТҪТ©ЎўҫьКВөИБмУтУҰУГ№г·әЎЈ№ӨТөЙПНЁіЈУГҪУҙҘ·ЁЦЖБтЛбЈ¬ЦчТӘФӯБПКЗБтМъҝуәНҝХЖшЎЈҪУҙҘ·ЁЦЖБтЛбөДЙъІъ№эіМҙуЦВҝЙ·ЦОӘИэёцҪЧ¶ОЈә¶юСх»ҜБтөДЦЖИЎәНҫ»»ҜЈ»¶юСх»ҜБтЧӘ»ҜОӘИэСх»ҜБтЈ»ИэСх»ҜБтөДОьКХәНБтЛбөДЙъіЙЎЈОӘБЛ·АЦ№»·ҫіОЫИҫІў¶ФОІЖшҪшРРЧЫәПАыУГЈ¬БтЛбі§іЈУГ°ұЛ®ОьКХОІЖшөДSO2ЎўSO3өИЖшМеЈ¬ФЩПтОьКХТәЦРјУИлЕЁБтЛбЈ¬ТФЦЖИЎёЯЕЁ¶ИөДSO2ј°ЈЁNH4Ј©2SO4әНNH4HSO4№ММеЎЈОӘБЛІв¶ЁЙПКцЈЁNH4Ј©2SO4әНNH4HSO4№ММе»мәПОпөДЧйіЙЈ¬ПЦіЖИЎёГСщЖ·ЛД·ЭЈ¬·ЦұрјУИлПаН¬ЕЁ¶ИөДNaOHИЬТә50.00mLЈ¬јУИИЦБ120ЎжЧуУТЈ¬К№°ұЖшИ«ІҝТЭіц[ЈЁNH4Ј©2SO4әНNH4HSO4өД·ЦҪвОВ¶ИҫщёЯУЪ200Ўж]Ј¬ІвөГУР№ШКөСйКэҫЭИзПВЈЁұкЧјЧҙҝцЈ©Јә
КөСй | СщЖ·өДЦКБҝ/g | NaOHИЬТәөДМе»э/mL | °ұЖшөДМе»э/LЈЁұкЧјЧҙҝцЈ© |
1 | 7.24 | 50.00 | 1.792 |
2 | 14.48 | 50.00 | 3.584 |
3 | 21.72 | 50.00 | 4.032 |
4 | 36.20 | 50.00 | 2.240 |
ЈЁ1Ј©УЙ1ЧйКэҫЭЦұҪУНЖІвЈә1.81gСщЖ·ҪшРРН¬СщКөСйКұЈ¬ЙъіЙ°ұЖшөДМе»эЈЁұкЧјЧҙҝцЈ©ОӘ___LЎЈ
ЈЁ2Ј©КФјЖЛгёГ»мәПОпЦРЈЁNH4Ј©2SO4әН NH4HSO4өДОпЦКөДБҝЦ®ұИОӘ___ЎЈ
ЈЁ3Ј©ЗуЛщУГNaOHИЬТәөДОпЦКөДБҝЕЁ¶И___mol/LЎЈ
Ўҫҙр°ёЎҝ0.448 1ЎГ2 6
ЎҫҪвОцЎҝ
ЈЁNH4Ј©2SO4әН NH4HSO4өД»мәПТәЦРјУИлЗвСх»ҜДЖЈ¬ТАҙО·ўЙъөД·ҙУҰКЗ2NH4HSO4+2NaOH=ЈЁNH4Ј©2SO4+Na2SO4+2H2OЎўЈЁNH4Ј©2SO4+2NaOH= Na2SO4+2H2O+2NH3ЎЈ
ЈЁ1Ј©ёщҫЭКөСй1Ўў2ҝЙЦӘЈ¬ФцјУСщЖ·өДЦКБҝЈ¬·ЕіцөД°ұЖшФц¶аЈ¬ЛөГчКөСй1ЦРЗвСх»ҜДЖ№эБҝЈ¬7.24gСщЖ·ЦРөДп§ёщАлЧУИ«ІҝЙъіЙ°ұЖш·ЕіцЈ¬ИфјУИл1.81gСщЖ·Ј¬п§ёщАлЧУТІДЬИ«ІҝЙъіЙ°ұЖш·ЕіцЈ¬Йи·Еіц°ұЖшөДМе»эКЗVLЈ¬Фт![]() Ј¬V=0.448LЎЈ
Ј¬V=0.448LЎЈ
ЈЁ2Ј©КөСй1ЦРЈ¬7.24gСщЖ·ЦРөДп§ёщАлЧУИ«ІҝЙъіЙ°ұЖш·ЕіцЈ¬ЙиСщЖ·ЦРөДЈЁNH4Ј©2SO4әН NH4HSO4ОпЦКөДБҝ·ЦұрКЗxmolЎўymolЈ¬ёщҫЭөӘФӘЛШКШәгЈ¬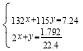 Ј¬ҪвөГx=0.02molЎўy=0.04molЈ¬
Ј¬ҪвөГx=0.02molЎўy=0.04molЈ¬![]() Ј»
Ј»
ЈЁ2Ј©УЙКөСй3Ўў4ҝЙТФҝҙіцЈ¬КөСй3ФЩФцҙуСщЖ·өДЦКБҝЈ¬ЙъіЙ°ұЖшөДМе»эјхРЎЈ¬ЛөГчЗвСх»ҜДЖОпЦКөДБҝІ»ЧгЈ¬Йи36.2gСщЖ·ЦРNH4HSO4ОпЦКөДБҝОӘamolЈ¬Фт![]() Ј¬a=0.2molЈ¬ПИ·ўЙъ·ҙУҰH++OH-=H2OЈ¬ФтёГ·ҙУҰПыәДЗвСх»ҜДЖөДОпЦКөДБҝОӘ0.2molЈ¬ФЩ·ўЙъNH4++OH-
Ј¬a=0.2molЈ¬ПИ·ўЙъ·ҙУҰH++OH-=H2OЈ¬ФтёГ·ҙУҰПыәДЗвСх»ҜДЖөДОпЦКөДБҝОӘ0.2molЈ¬ФЩ·ўЙъNH4++OH-![]() Ј¬ёщҫЭ°ұЖшөДМе»эОӘ2.24LЈ¬ёГ·ҙУҰПыәДЗвСх»ҜДЖөДОпЦКөДБҝОӘ0.1molЈ¬ЛщТФ50mLЗвСх»ҜДЖИЬТәә¬УРЗвСх»ҜДЖөДОпЦКөДБҝКЗ0.3molЈ¬ЗвСх»ҜДЖИЬТәөДЕЁ¶ИКЗ
Ј¬ёщҫЭ°ұЖшөДМе»эОӘ2.24LЈ¬ёГ·ҙУҰПыәДЗвСх»ҜДЖөДОпЦКөДБҝОӘ0.1molЈ¬ЛщТФ50mLЗвСх»ҜДЖИЬТәә¬УРЗвСх»ҜДЖөДОпЦКөДБҝКЗ0.3molЈ¬ЗвСх»ҜДЖИЬТәөДЕЁ¶ИКЗ![]() 6mol/LЎЈ
6mol/LЎЈ


 ЦұНЁ№уЦЭГыРЈЦЬІвФВҝјЦұНЁГыРЈПөБРҙр°ё
ЦұНЁ№уЦЭГыРЈЦЬІвФВҝјЦұНЁГыРЈПөБРҙр°ё ЕаУЕИэәГЙъПөБРҙр°ё
ЕаУЕИэәГЙъПөБРҙр°ё
| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝH2O2КЗКөСйКТіЈјыөДЗҝСх»ҜјБЈ¬ФЪТҪБЖЙПҝЙУГЧчПы¶ҫјБөИЎЈ
ЈЁ1Ј©Т»ЦЦХэФЪҝӘ·ўөДАыУГO2әНH2OЧчФӯБПНЁ№э»ҜәПЦЖИЎH2O2өД·Ҫ·ЁЈ¬ЖдФӯАнИзНјЛщКҫЎЈёГ·Ҫ·ЁЦЖИЎH2O2өДЧЬ»ҜС§·ҙУҰ·ҪіМКҪОӘ____ЎЈ
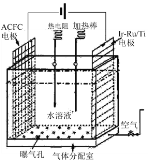
ЈЁ2Ј©ОӘМҪҫҝНвҪзМхјю¶ФH2O2·ЦҪв»ҜС§·ҙУҰЛЩВКөДУ°ПмЈ¬Па№ШКөСйЙијЖИзПВұнЛщКҫЈә
КФ№Ь ұаәЕ | КөСйДҝөД | H2O2ИЬТә | ОВ¶И | Л®өД Ме»э/mL | FeCl3ИЬТәМе»э/mL | |
ЦКБҝ ·ЦКэ | Ме»э/mL | |||||
ўс | ОӘұаәЕўтКөСйІОХХ | 12% | 5Ј®0 | іЈОВ | 0 | 0 |
ўт | ОВ¶И¶Ф·ҙУҰЛЩВКөДУ°Пм | ЈЁ Ј© | 5Ј®0 | 60Ўж | 0 | 0 |
ўу | ОӘұаәЕўфКөСйІОХХ | 4Ј®0% | 5Ј®0 | іЈОВ | ЈЁ Ј© | 0 |
ўф | ЈЁ Ј© | 4Ј®0% | 5Ј®0 | іЈОВ | 0 | 1Ј®0 |
МоРҙұнЦРИұЙЩөДДЪИЭЈәўт_______Ј»ўу__________Ј»ўф_________ЎЈ
ЈЁ3Ј©УЙІ¬ЈЁPtО»УЪЧуұЯЈ©әНҪрЈЁAuО»УЪУТұЯЈ©ЧйіЙөДДЙГЧ°ф·ЕИлH2O2ИЬТәЦРЈЁИзНјЈ©Ј¬ДЙГЧ°фҪ«·ўЙъ¶ЁПтТЖ¶ҜЎЈ
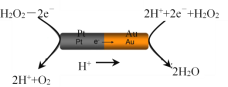
ФтЈәAuТ»ІаОӘөзіШөД____ј«ЈЁСЎМоЈәЎ°ХэЎұ»тЎ°ёәЎұЈ©Ј»ДЙГЧ°фПт____ЈЁСЎМоЈәЎ°ЧуЎұ»тЎ°УТЎұЈ©ТЖ¶ҜЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝЎ°ТФ·ПЦО·ПЎұКЗ»щУЪЎ°ВМЙ«»ҜС§Ўұ№ЫДоЦОАнОЫИҫөДЛјВ·ЎЈУГ№ӨТө·ПјоФьЈЁЦчТӘіЙ·ЦОӘNa2CO3Ј©ОьКХСМЖшЦРөДSO2Ј¬өГөҪСЗБтЛбДЖЈЁNa2SO3Ј©ҙЦЖ·ЎЈЖдБчіМИзПВЈ¬ПВБРЛө·ЁХэИ·өДКЗ
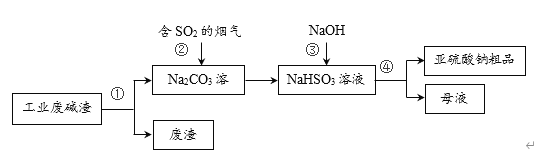
A.ІЩЧчўЩЎўўЬҫщОӘ№эВЛ
B.ІҪЦиўЪЦР·ўЙъБЛЦГ»»·ҙУҰ
C.ІҪЦиўЫ·ўЙъөД·ҙУҰОӘЈәNaHSO3Ј«NaOH = Na2SO3Ј«H2O
D.СЗБтЛбДЖҙЦЖ·ЦРІ»ҝЙДЬә¬УРNa2SO4
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝДіС§П°РЎЧйУГПВНјЧ°ЦГСРҫҝSO2өДРФЦКЎЈ
| РтәЕ | X | КөСйПЦПу |
ўс | ЧПЙ«КҜИпИЬТә | ПЦПуa | |
ўт | Ж·әмИЬТә | ИЬТәУЙәмЙ«ұдОӘОЮЙ«Ј¬јУИИәуУЦ»ЦёҙФӯАҙөДСХЙ« | |
ўу | ЛбРФKMnO4ИЬТә | ИЬТәУЙЧПЙ«ұдОӘОЮЙ« |
Зл»ШҙрЈә
ЈЁ1Ј©КөСйўсЦРЈ¬ПЦПуaКЗ______ЎЈ
ЈЁ2Ј©ёщҫЭКөСйўтЈ¬НЖ¶ПSO2өД»ҜС§РФЦККЗ______ЎЈ
ЈЁ3Ј©ёщҫЭКөСйўуЈ¬НЖ¶ПОЮЙ«ИЬТәЦРЛщә¬өДАлЧУКЗK+ЎўMn2+ЎўH+әН______ЎЈ
ЈЁ4Ј©ҪбәПАлЧУ·ҪіМКҪЛөГчКөСйЦРNaOHИЬТәөДЧчУГКЗ______ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝ(1)УРТ»ЖҝОЮЙ«іОЗеИЬТәЈ¬ЖдЦРҝЙДЬә¬H+ЎўNa+ЎўMg2+ЎўBa2+ЎўClЎўSO42ЎўCO32АлЧУЎЈПЦҪшРРТФПВКөСйЈә
AЎўУГpHКФЦҪјмСйИЬТәЈ¬·ўПЦИЬТәіКЗҝЛбРФЈ»
BЎўИЎІҝ·ЦИЬТәЦрөОјУИлNaOHИЬТәЈ¬К№ИЬТәУЙЛбРФұдОӘјоРФЈ¬ОЮіБөнІъЙъЈ»
CЎўИЎЙЩБҝBЦРөДјоРФИЬТәЈ¬өОјУNa2CO3ИЬТәЈ¬УР°ЧЙ«іБөнІъЙъЎЈ
ўЩёщҫЭЙПКцКВКөИ·¶ЁЈәёГИЬТәЦРҝП¶ЁҙжФЪөДАлЧУУР_________________________Ј»
ҝП¶ЁІ»ҙжФЪөДАлЧУУР___________________________ЎЈ
ўЪРҙіцCЦР·ўЙъ·ҙУҰөДАлЧУ·ҪіМКҪ________________________________ЎЈ
(2)ўЩ»№ФӯМъ·ЫУлёЯОВЛ®ХфЖш·ҙУҰөД»ҜС§·ҪіМКҪЈә_____________________________Ј»
ўЪіэИҘMg·ЫЦРөДAl·ЫөДКФјБКЗ__________________Ј¬·ҙУҰөДАлЧУ·ҪіМКҪОӘЈә___________________________________Ј»
(3)ёЯМъЛбДЖЈЁNa2FeO4Ј©ҫЯУРЗҝСх»ҜРФЈ¬ҝЙ¶ФЧФАҙЛ®ҪшРРПы¶ҫЎўҫ»»ҜЎЈёЯМъЛбДЖҝЙУГЗвСх»ҜМъәНҙОВИЛбДЖФЪјоРФҪйЦКЦР·ҙУҰөГөҪЈ¬ЗлІ№ідІўЕдЖҪПВГжАлЧУ·ҪіМКҪЎЈ
____Fe(OH)3 +____ClOЈӯ+____OHЈӯ =__FeO42ЈӯЈ«___ClЈӯ+_____ _______
(4)ФЪ·ҙУҰ11P+15CuSO4+24H2O=5Cu3P+6H3PO4+15H2SO4ЦРЈ¬Сх»ҜјБКЗ___________Ј»
өұУР2mol H3PO4ЙъіЙЈ¬ЧӘТЖөДөзЧУөДОпЦКөДБҝОӘ__________________.
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБРЛө·ЁІ»ХэИ·өДКЗЈЁ Ј©
A.35ClәН37Cl»ҘОӘН¬О»ЛШ
B.ТТЛбәНУНЛбЈЁC17H33COOHЈ©»ҘОӘН¬ПөОп
C.әмБЧәН°ЧБЧ»ҘОӘН¬ЛШТмРОМе
D.ТТИ©әН»·СхТТНй(![]() )»ҘОӘН¬·ЦТм№№Ме
)»ҘОӘН¬·ЦТм№№Ме
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝБтЛбКЗј«ЖдЦШТӘөД»Ҝ№ӨФӯБПЈ¬ФЪ№ӨТөЎўЕ©ТөЎўТҪТ©ЎўҫьКВөИБмУтУҰУГ№г·әЎЈ№ӨТөЙПНЁіЈУГҪУҙҘ·ЁЦЖБтЛбЈ¬ЦчТӘФӯБПКЗБтМъҝуәНҝХЖшЎЈҪУҙҘ·ЁЦЖБтЛбөДЙъІъ№эіМҙуЦВҝЙ·ЦОӘИэёцҪЧ¶ОЈә¶юСх»ҜБтөДЦЖИЎәНҫ»»ҜЈ»¶юСх»ҜБтЧӘ»ҜОӘИэСх»ҜБтЈ»ИэСх»ҜБтөДОьКХәНБтЛбөДЙъіЙЎЈОӘБЛ·АЦ№»·ҫіОЫИҫІў¶ФОІЖшҪшРРЧЫәПАыУГЈ¬БтЛбі§іЈУГ°ұЛ®ОьКХОІЖшөДSO2ЎўSO3өИЖшМеЈ¬ФЩПтОьКХТәЦРјУИлЕЁБтЛбЈ¬ТФЦЖИЎёЯЕЁ¶ИөДSO2ј°ЈЁNH4Ј©2SO4әНNH4HSO4№ММеЎЈОӘБЛІв¶ЁЙПКцЈЁNH4Ј©2SO4әНNH4HSO4№ММе»мәПОпөДЧйіЙЈ¬ПЦіЖИЎёГСщЖ·ЛД·ЭЈ¬·ЦұрјУИлПаН¬ЕЁ¶ИөДNaOHИЬТә50.00mLЈ¬јУИИЦБ120ЎжЧуУТЈ¬К№°ұЖшИ«ІҝТЭіц[ЈЁNH4Ј©2SO4әНNH4HSO4өД·ЦҪвОВ¶ИҫщёЯУЪ200Ўж]Ј¬ІвөГУР№ШКөСйКэҫЭИзПВЈЁұкЧјЧҙҝцЈ©Јә
КөСй | СщЖ·өДЦКБҝ/g | NaOHИЬТәөДМе»э/mL | °ұЖшөДМе»э/LЈЁұкЧјЧҙҝцЈ© |
1 | 7.24 | 50.00 | 1.792 |
2 | 14.48 | 50.00 | 3.584 |
3 | 21.72 | 50.00 | 4.032 |
4 | 36.20 | 50.00 | 2.240 |
ЈЁ1Ј©УЙ1ЧйКэҫЭЦұҪУНЖІвЈә1.81gСщЖ·ҪшРРН¬СщКөСйКұЈ¬ЙъіЙ°ұЖшөДМе»эЈЁұкЧјЧҙҝцЈ©ОӘ___LЎЈ
ЈЁ2Ј©КФјЖЛгёГ»мәПОпЦРЈЁNH4Ј©2SO4әН NH4HSO4өДОпЦКөДБҝЦ®ұИОӘ___ЎЈ
ЈЁ3Ј©ЗуЛщУГNaOHИЬТәөДОпЦКөДБҝЕЁ¶И___mol/LЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝДі№ММеXҝЙДЬә¬УРNa2O2ЎўFe2O3ЎўAl2O3ЎўSiO2ЎўK2SO4ЎўNa2SO3ЎўNH4NO3ЎўMgCl2ЦРөДТ»ЦЦ»тјёЦЦОпЦКЈ¬ҪшРРИзПВКөСйТФИ·¶ЁЖдЧйіЙЈә
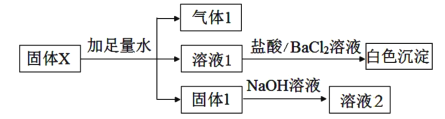
ПВБРЛө·ЁІ»ХэИ·өДКЗЈЁ Ј©
A.ИЬТә1ЦРІ»ҝЙДЬә¬УРCl-
B.ЖшМе1ҝЙДЬКЗ¶юЦЦЖшМеөД»мәПОп
C.№ММе1ҝЙДЬКЗ¶юЦЦ№ММеөД»мәПОп
D.№ММеXЦРЈ¬K2SO4әНNa2SO3БҪЦЦОпЦКЦБЙЩә¬Т»ЦЦ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПЦУРҪрКфөҘЦКAЎўBЎўCәНЖшМејЧЎўТТЎўұыТФј°ОпЦКDЎўEЎўFЎўGЎўHЈ¬ЛьГЗЦ®јдөДПа»ҘЧӘ»Ҝ№ШПөИзНјЛщКҫЈЁНјЦРУРР©·ҙУҰөДЙъіЙОпәН·ҙУҰөДМхјюГ»УРұкіцЈ©ЎЈЗлёщҫЭТФЙПРЕПўНкіЙПВБРёчМвЈә
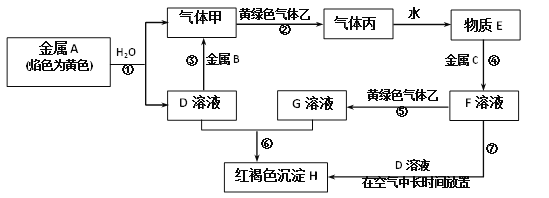
(1)РҙіцПВБРОпЦКөД»ҜС§КҪЈәB_______Ўўұы_______ЎЈ
(2)Рҙіц»ЖВМЙ«ЖшМеТТөДТ»ЦЦУГНҫ_______Ј¬·ҙУҰ№эіМўЯҝЙДЬ№ЫІмөҪөДКөСйПЦПуКЗ________ЎЈ
(3)Рҙіц·ҙУҰўЯЦРЙжј°өД»ҜС§·ҙУҰ·ҪіМКҪЈә_______Ўў________ЎЈ
(4)Рҙіц·ҙУҰўЭөДАлЧУ·ҪіМКҪ____________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com