
 ij��ѧ�о�С��Ϊ�ⶨNa2O2��Ʒ������Na2O���ʣ��Ĵ��ȣ����������ʵ�鷽������̽����
ij��ѧ�о�С��Ϊ�ⶨNa2O2��Ʒ������Na2O���ʣ��Ĵ��ȣ����������ʵ�鷽������̽����| ʵ����� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �����������mL�� | 23.00 | 24.98 | 25.00 | 25.02 |
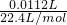 =0.0005mol�����������Ϊ0.0005mol��2=0.001mol������Ϊ0.001mol��78g/mol=0.078g���ʹ������Ƶ���������Ϊ
=0.0005mol�����������Ϊ0.0005mol��2=0.001mol������Ϊ0.001mol��78g/mol=0.078g���ʹ������Ƶ���������Ϊ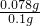 ��100%=78%��
��100%=78%��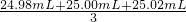 =25.00mL��
=25.00mL��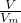 �������������ʵ�������������������Ƶ����ʵ���������������Ƶ�������������������������㣻
�������������ʵ�������������������Ƶ����ʵ���������������Ƶ�������������������������㣻


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2011?����һģ��ij��ѧ�о�С��Ϊ�ⶨNa2O2��Ʒ������Na2O���ʣ��Ĵ��ȣ����������ʵ�鷽������̽����
��2011?����һģ��ij��ѧ�о�С��Ϊ�ⶨNa2O2��Ʒ������Na2O���ʣ��Ĵ��ȣ����������ʵ�鷽������̽����| ʵ����� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �����������mL�� | 23.00 | 24.98 | 25.00 | 25.02 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ�о�С��Ϊ�ⶨNa2O2��Ʒ������Na2O���ʣ��Ĵ��ȣ����������ʵ�鷽������̽����
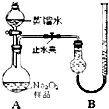
[ʵ��һ] ȡ��Ʒ0. 100 g������ͼ��ʾʵ��װ�ý��вⶨ��
���г�װ��ʡ�ԣ�
��1�����װ��A�����Եķ����� ��
��2������ʵ������й���������11.20 mL��������ɱ�
״����������Ʒ��Na2O2�Ĵ���Ϊ ��
[ʵ���] ��Ʒ����ˮ��������ζ�
��3��ȡһ������Ʒ����ˮ�����Ƴ�250 mL��Һ������ʱ�����õ��IJ������������ձ��Ͳ������⣬�����õ� ��
��4��ȡ����������Һ��25.00 mL����һ��Ũ�ȵı�����ζ���ƽ��ʵ���¼���±���
| ʵ����� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �����������mL�� | 23.00 | 24.98 | 25.00 | 25.02 |
�ɱ������ݿ�֪�������������ƽ��ֵΪ mL�����ζ�ǰ������������Һ��ϴ��ƿ����ʵ��ⶨ���������Ӱ���� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
[ʵ�����] ʵ�����Աȷ���
��5��С��ͬѧ�Ա�����ʵ�鷢�֣�ʵ��һ��õ�Na2O2��Ʒ�Ĵ��ȱ�����ֵ����ƫС������������С��ͬѧ��Ϊ������ƫ����ɲ���ʧ���װ��ȱ������ģ������Ƿ�Ӧԭ���ϵ�ԭ�������Ϸ��֣�Na2O2��ˮ��Ӧ������H2O2δ��ȫ�ֽ⡣
��д��Na2O2��ˮ����H2O2�Ļ�ѧ����ʽ ��
������ʵ��һ�ԼӸĽ�������ʹ�ⶨ����ӽ�����ֵ��д���Ľ�������
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������ʡ�����е���ʮ����ѧ������ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��15�֣�ij��ѧ�о�С��Ϊ�ⶨNa2O2��Ʒ������Na2O���ʣ��Ĵ��ȣ����������ʵ�鷽������̽����
[ʵ��һ]ȡ��Ʒ0.100 g������ͼ��ʾʵ��װ�ý��вⶨ�����г�װ��ʡ�ԣ�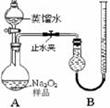
��1�����װ��A�����Եķ����� ����������������������������
��2������ʵ������й���������11.20 mL��������ɱ�״����������Ʒ��Na2O2�Ĵ���Ϊ������ ������������������������
[ʵ���]��Ʒ����ˮ��������ζ�
��3��ȡһ������Ʒ����ˮ��ϡ����250 mL������ʱ�����õ��IJ������������ձ��Ͳ������⣬�����õ�������������������������
��4��ȡ����������Һ��25.00 mL����һ��Ũ�ȵı�����ζ���ƽ��ʵ���¼���±���
| ʵ����� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �����������mL�� | 23.00 | 24.98 | 25.00 | 25.02 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�츣��ʡ�����и�����ͨ���б�ҵ��������飨���ۣ���ѧ���� ���ͣ�ʵ����
ij��ѧ�о�С��Ϊ�ⶨNa2O2��Ʒ������Na2O���ʣ��Ĵ��ȣ����������ʵ�鷽������̽����
[ʵ��һ] ȡ��Ʒ0. 100 g������ͼ��ʾʵ��װ�ý��вⶨ��
���г�װ��ʡ�ԣ�
��1�����װ��A�����Եķ����� ��
��2������ʵ������й���������11.20 mL��������ɱ�
״����������Ʒ��Na2O2�Ĵ���Ϊ ��
[ʵ���] �� Ʒ����ˮ��������ζ�
Ʒ����ˮ��������ζ�
��3��ȡһ������Ʒ����ˮ�����Ƴ�250 mL��Һ������ʱ�����õ��IJ������������ձ��Ͳ������⣬�����õ� ��
��4��ȡ����������Һ��25.00 mL����һ��Ũ�ȵı�����ζ���ƽ��ʵ���¼���±���
| ʵ����� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �����������mL�� | 23.00 | 24.98 | 25.00 | 25.02 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡ�����и�����ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��15�֣�ij��ѧ�о�С��Ϊ�ⶨNa2O2��Ʒ������Na2O���ʣ��Ĵ��ȣ����������ʵ�鷽������̽����
[ʵ��һ]ȡ��Ʒ0.100 g������ͼ��ʾʵ��װ�ý��вⶨ�����г�װ��ʡ�ԣ�
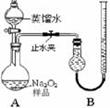
��1�����װ��A�����Եķ����� ����������������������������
��2������ʵ������й���������11.20 mL��������ɱ�״����������Ʒ��Na2O2�Ĵ���Ϊ������ ������������������������
[ʵ���]��Ʒ����ˮ��������ζ�
��3��ȡһ������Ʒ����ˮ��ϡ����250 mL������ʱ�����õ��IJ������������ձ��Ͳ������⣬�����õ�������������������������
��4��ȡ����������Һ��25.00 mL����һ��Ũ�ȵı�����ζ���ƽ��ʵ���¼���±���
|
ʵ����� |
��һ�� |
�ڶ��� |
������ |
���Ĵ� |
|
�����������mL�� |
23.00 |
24.98 |
25.00 |
25.02 |
�ɱ������ݿ�֪�������������ƽ��ֵΪ������������ ������mL�����ζ�ǰ������������Һ��ϴ��ƿ���Բⶨ�������������Ӱ���������������������� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
[ʵ�����]ʵ�����Աȷ���
��5��С��ͬѧ�Ա�����ʵ�鷢�֣�ʵ��һ��õ�Na2O2��Ʒ�Ĵ��ȱ�����ֵ����ƫС������������С��ͬѧ��Ϊ������ƫ����ɲ���ʧ���װ��ȱ������ģ������Ƿ�Ӧԭ���ϵ�ԭ�������Ϸ��֣�
Na2O2��ˮ��Ӧ������H2O2δ��ȫ�ֽ⡣
��д��Na2O2��ˮ����H2O2�Ļ�ѧ����ʽ����������������������������������������
������ʵ��һ�ԼӸĽ�������ʹ�ⶨ����ӽ�����ֵ��д���Ľ�������������������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com