
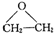
 ���÷�Ӧ��ԭ��������Ϊ100%����Ӧ�Ļ�ѧ����ʽΪ2CH2=CH2+O2$��_{��}^{Ag}$
���÷�Ӧ��ԭ��������Ϊ100%����Ӧ�Ļ�ѧ����ʽΪ2CH2=CH2+O2$��_{��}^{Ag}$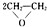 ��
�� ���� ��1��̼�⻯�������C��HԪ�أ�
��2���Ҵ��ڴ������±���������XΪ��ȩ��
��3���Ҵ������������ظ������Һ��Ӧ����ֱ��������Y��YΪ���ᣬWΪ����������
��4������������ԣ�����ʯ����Һ��̼������Һ���飻
��5����ϩ����̼̼˫��������ˮ�����ӳɷ�Ӧ�����Ҵ�����ϩ��������Ӧ���� ����������غ���ƽ����ʽ��
����������غ���ƽ����ʽ��
��� �⣺��1��̼�⻯�������C��HԪ�أ����������࣬�ʴ�Ϊ������
��2���Ҵ��ڴ������±���������XΪ��ȩ���ṹ��ʽΪCH3CHO���ʴ�Ϊ��CH3CHO��
��3���Ҵ������������ظ������Һ��Ӧ����ֱ��������Y��YΪ���ᣬ���еĹ�����Ϊ�Ȼ���WΪ�����������Ҵ�������֮����Է���������Ӧ��
CH3CH2OH+CH3COOH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
�ʴ�Ϊ���Ȼ���CH3CH2OH+CH3COOH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
��4������������ԣ�����ʯ����Һ��̼������Һ���飬�ʴ�Ϊ��ʯ����Һ��̼������Һ��
��5����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����ϩ�ܱ������������ɻ������飬���������غ㶨�ɸ÷�ӦΪ2CH2=CH2+O2$��_{��}^{Ag}$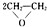 ��
��
�ʴ�Ϊ���ӳɷ�Ӧ��2CH2=CH2+O2$��_{��}^{Ag}$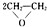 ��
��
���� ���⿼���л���Ľṹ�����ʣ�Ϊ��Ƶ���㣬���չ����������ʵĹ�ϵΪ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע���Ҵ������ʼ�Ӧ�ã��ۺ��Խ�ǿ����Ŀ�ѶȲ���


 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
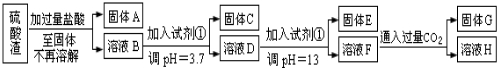
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����⻯�������H+��������λ����ϣ�����Ľṹʽ
�����⻯�������H+��������λ����ϣ�����Ľṹʽ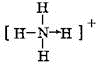 ��
�� ��1mol O22+�к��еĦм�Ϊ2mol��
��1mol O22+�к��еĦм�Ϊ2mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
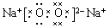
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��̬��ԭ�Ӻ���۵��ӵĹ����ʾʽ�� | |
| B�� | HClO�ĽṹʽΪH-Cl-O | |
| C�� | �õ���ʽ��ʾNaCl���γɹ��̣� | |
| D�� | F-�Ľṹʾ��ͼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | A | B | C | D |
| n��CO2����mol�� | 6 | 4 | 3 | 2 |
| n����������mol�� | 2 | 3 | 2 | 1 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com