
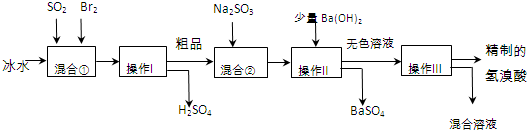
���� ��Ϣ��з�����Ӧ SO2+Br2+2H2O=H2SO4+2HBr��������������������������Ĵ�Ʒ�����壩����Ϣ��м���Na2SO3��ԭ��Ʒ�е�Br2�����ᷴӦ����SO42- ����������������˵����ᱵ��������ɫ��Һ��������õ����µ������ᣬ
��1��Br2����ǿ�����ԣ�����Һ�н�SO2����ΪH2SO4����������ԭΪHBr��
��2���ɹ������̿�֪����������������Һ�壬Ӧ�ǹ��ˣ�������Ϊ���ܵ���Һ��ֵķ��룬Ӧ����������һ�������ڷе㲻ͬ�Ļ��ܵ�Һ��ķ��룻
��3����Ʒ�п��ܺ���Ϊ��Ӧ��Br2��Ӧ��ȥBr2��
��4����KSCN��Һ����Fe3+���μ�KSCN��Һ����Һ���Ѫ��ɫ���ɹ������̿�֪����Һ�п��ܺ���Br2��������CCl4��ȡ�������飻
��� �⣺��Ϣ��з�����Ӧ SO2+Br2+2H2O=H2SO4+2HBr��������������������������Ĵ�Ʒ�����壩����Ϣ��м���Na2SO3��ԭ��Ʒ�е�Br2�����ᷴӦ����SO42- ����������������˵����ᱵ��������ɫ��Һ��������õ����µ������ᣬ
��1��Br2����ǿ�����ԣ�����Һ�н�SO2����ΪH2SO4����������ԭΪHBr����Ӧ����ʽΪSO2+Br2+2H2O=2HBr+H2SO4��
�ʴ�Ϊ��SO2+Br2+2H2O=2HBr+H2SO4��
��2���ɹ������̿�֪����������������Һ�壬Ӧ�ǹ��ˣ�������Ϊ���ܵ���Һ��ֵķ��룬Ӧ����������һ�������ڷе㲻ͬ�Ļ��ܵ�Һ��ķ��룬
�ʴ�Ϊ�����ˣ�����d��
��3����Ʒ�п��ܺ���Ϊ��Ӧ��Br2������Na2SO3����ȥ��Ʒ��δ��Ӧ����壬
�ʴ�Ϊ����ȥ��Ʒ��δ��Ӧ����壻
��4����KSCN��Һ����Fe3+��ȡ������Һ�μ�KSCN��Һ����Һ���Ѫ��ɫ��˵��������ʵ���ɫ����Ϊ��Fe3+���ɹ������̿�֪����Һ�п��ܺ���Br2��������CCl4��ȡ�������飬ȡ������Һ�������÷ֲ㣬�²�ʳȺ�ɫ��˵��������ʵ���ɫ����Ϊ��Br2��
�ʴ�Ϊ��KSCN��Һ������Br2��CCl4��
���� ���Ʊ�������Ϊ���壬����ѧ���Թ������̵����⡢���ʵķ����ᴿ�Ȼ������������Ӽ��顢�������ʵȣ��Ѷ��еȣ��Ƕ�֪ʶ���ۺ����ã���ѧ��������ʵ�Ļ���֪ʶ���������֪ʶ��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
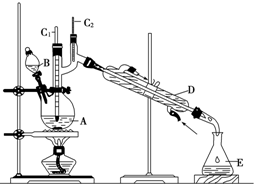 ����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С����������װ�úϳ�����ȩ��
����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С����������װ�úϳ�����ȩ��| �е�/�� | �ܶ�/��g•cm-3�� | ˮ���ܽ��� | |
| ������ | 117.2 | 0.810 9 | �� |
| ����ȩ | 75.7 | 0.801 7 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| �¶� | ʱ��/min n/mol | 0 | 10 | 20 | 40 | 50 |
| T1 | n��N2�� | 0 | 0.20 | 0.35 | 0.40 | 0.40 |
| T2 | n��N2�� | 0 | 0.25 | �� | 0.30 | 0.30 |
| A�� | T1�¶��£�CH4��ƽ��ת����Ϊ50% | |
| B�� | T1��T2 | |
| C�� | a��0 | |
| D�� | T2ʱ��Ӧ��ƽ�ⳣ������T1ʱ��Ӧ��ƽ�ⳣ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com