
(1)������������ƽ��������ʱ�������̷���һ��1 g �����룬����λ������ͼ��ʾ��

(2)���ƺõ��η�����ƿ�У�������ˮ�ܽ⣬��Һ��ɫ���ټ���ָʾ��_________________(�ӷ�̪��ʯ����ѡ��)1��2�Σ���NaOH��Һ�ζ����յ㣬������________________________��
(3)������������ʵ�飬��ȡ������������ͬ����д�±���
ʵ���� | ��ʽ�ε�����/g | ����NaOH��Һ���/mL |
1 |
| 18.2 |
2 |
| 17.1 |
3 |
| 16.9 |
(4)�ζ������ϴ���ǵ�________��ʵ�飬����������Ŀ���ԭ����(ֻ��д���ּ���)��
��_____________________________________________________________��
��_____________________________________________________________��
��_____________________________________________________________��
(5)NaOH��Һ�����ʵ���Ũ��Ϊ___________________(ֻ�г�����ʽ������������)��
(2)��̪ ��ɫ���dz��ɫ����0.5 min�ڲ���ɫ
(3)0.4 0.4 0.4
(4)1 ��ʢװNaOH��Һ�ĵζ���δ��NaOH��Һ��ϴ��ֻ��ˮϴ����������NaOH��Һ���ƫ�ڵζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ���۵ζ��յ��жϲ���ʹ��Һ�ʺ�ɫ���ܵζ�ʱ�еζ�Һ������ƿ�⣻�ݶ��յ����ʱ���������ӵȵȡ�(��д��������)
(5)0.12 mol��L-1
������(1)������ƽ�ij���ԭ���Ǹܸ�ƽ��ԭ������������ƽ����ʱ�������ڱ�����������������Ǽӵ������ϵģ��������ԭ��Ϊ�������롣�ֽ�����������������ϣ���������������ϣ������ڱ����ָʾ������Ϊ0.6 g����m(������)+m(����)=m(����)����m(������)+0.6 g=1 g��m(������)=0.4 g��
(2)��ζ��յ�ʱ��Һ��pHԼΪ9.1����̪�ı�ɫ��Χ��pH=8��10����ʯ��ı�ɫ��Χ��pH=5��8����Ӧѡ�÷�̪��ָʾ���������Ǽ�ζ��������ʣ��ʵζ��յ������Ӧ����ɫ���dz��ɫ����0.5 min�ڲ���ɫ��
(3)�������ӦΪ(1)��m(������)=0.4 g��
(4)�ӱȽϱ�����������NaOH��Һ��������Կ������������������������С������һ������ֵ�������������ֵ�нϴ��ƫ��ʵζ������ϴ��Ӧ�ǵ�һ�Ρ���һ��������ֵ���Դ��ڶ������Σ�����������Ŀ���ԭ���У���ʢװNaOH��Һ�ĵζ���δ��NaOH��Һ��ϴ��ֻ��ˮϴ����������NaOH��Һ���ƫ�ڵζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ���۵ζ��յ��жϲ���ʹ��Һ�ʺ�ɫ���ܵζ�ʱ�еζ�Һ������ƿ�⣻�ݶ��յ����ʱ���������ӵȵȡ�
(5)������NaOH��Ӧʱ�����ʵ�����Ϊ1��1������n���=n���Σ�����c(NaOH)��
V(NaOH)=![]()
V(NaOH)=![]() ��10-3 L(���һ������NaOH��Һ��������ϴʲ���)
��10-3 L(���һ������NaOH��Һ��������ϴʲ���)
���ǣ�c(NaOH)=![]() mol��L-1=0.12 mol��L-1
mol��L-1=0.12 mol��L-1


 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| �� |
| ||
| �� |
| ����ø |
| �ƻ�ø |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
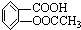 ����Է�������Ϊ180����
����Է�������Ϊ180����

| ����� | 0.1mol/L�������/mL | |
| �ζ�ǰ | �ζ��� | |
| 1 | 0.00 | 17.98 |
| 2 | 1.56 | 16.58 |
| 3 | 0.22 | 15.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

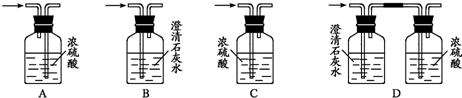
��1����װ��A��ȡ����������İ��������Թ���̼���εĻ�ѧʽ��__________________����ʯ�ҵ�������________________________________________________________________��
��2���������İ��������������ͨ��װ��B������Ϊ��ʯ�ޣ��У��þƾ���Ƽ��ȣ�
�ٰ��������Ļ�ѧ����ʽ��____________________________________���Թ��������Ϊ����ɫ���÷�Ӧ�Ļ�ѧ����ʽ��______________________________________________��
��ֹͣ��Ӧ�������ر�B������������һ��ʱ����Թܽ����ˮ��������һ�ֶ�����NO2��Է��������Ļ�����Թ���������ɫ��dz�����ϻ�ѧ����ʽ˵��ԭ��__________
_____________________________________________________________________��
��3����������������A�����İ����ֱ��a��b���ܽ�����ͨ�뵽װ��C�У���b���϶˵�ȼ����δ������ɫ���壺
��������ͨ����Ⱥ�˳����_______________����������__________________________��
�ڰ���ȼ�յĻ�ѧ����ʽ��________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ��ԭ���и߶��������¿���ѧ�Ծ� ���ͣ�ʵ����
��10�֣�ijУ��ѧ����С���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ������Ҵ��ľ��ơ��Ҵ����Ԫ�صIJⶨ������ʽ�IJⶨ�����ӽṹ�IJⶨ��
��1���������Ҵ��к�����ˮ������ֱ������ķ���������Ч��ȥˮ��ͨ�������м��� ��Ȼ������֤���Ҵ����ٺ�ˮ������һ���Լ����飬�����Լ��� ��
��2����ȼ���Ҵ���������ķ���ȷ������C��H��O����Ԫ�ء�
a��֤������HԪ�صIJ����� ��
b��֤������OԪ��ʱ����ȡ�õ�ʵ�������ǣ�CO2��������H2O�������� ��
��3��Ϊȷ���Ҵ��ķ���ʽ����ͨ��(2)��ȡ�Ҵ���ʵ��ʽ֮���Ƿ�����ٲⶨ�Ҵ�����Է�����������ȷ�������ʽ (���ǡ���)�������� ��
��4���ⶨ�Ҵ��ķ��ӽṹ
a�������ú�������Dzⶨ����ͨ�����ú���������� ���������շ壬����ȷ���Ҵ��Ľṹ��CH3CH2OH������CH3OCH3��(�C��H������C��C������C��O������O��H��)
b�������ú˴Ź����Dzⶨ���������Ҵ��ĺ˴Ź���������Ӧ�� �����շ塣
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com