
СђЫсбЧЬњЪЧживЊЕФбЧЬњбЮЃЌдкХЉвЕЩЯгУзїХЉвЉЃЌжївЊжЮаЁТѓКкЫыВЁЃЌЛЙПЩвдгУзїГ§ВнМСЃЛдкЙЄвЕЩЯгУгкШОЩЋЁЂжЦдьРЖКкФЋЫЎКЭФОВФЗРИЏЕШЁЃ
ЃЈ1ЃЉаТжЦЕФТЬЗЏЃЈFeSO4ЁЄ7H2OЃЉЪЧЧГТЬЩЋЕФЃЌЕЋдкПеЦјжаМЋвзБфГЩЛЦЩЋЛђЬњатЩЋЕФМюЪНСђЫсЬњ[Fe(OH)SO4]ЃЌаДГіИУЗДгІЕФЛЏбЇЗНГЬЪНЃК ЁЃ
ЃЈ2ЃЉвбжЊFeSO4дкВЛЭЌЬѕМўЯТЗжНтЕУЕНВњЮяВЛЭЌЃЌПЩФмЪЧFeOКЭSO3ЃЌвВПЩФмЪЧFe2O3ЁЂSO3КЭSO2ЃЛ
SO3ШлЕуЪЧ16.8ЁцЃЌЗаЕуЪЧ44.8ЁцЁЃ
ФГбаОПадбЇЯАаЁзщФтгУЯТСазАжУНјааЪЕбщЬНОПЁАдкМгШШЬѕМўЯТFeSO4ЕФЗжНтВњЮяЁБЁЃ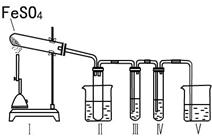
ЩЯЪізАжУЂѓКЭЂєгУРДМьбщЦјЬхВњЮяЁЃЪдЛиД№ЯТСаЮЪЬтЃК
ЂйЂђзАжУЩеБжаЫЎЕФЮТЖШгІПижЦдк ЃЈбЁЬюЁА0ЁцЁЂ25ЁцЁЂ50ЁцЁБЃЉЃЌзАжУЂђЕФзїгУЪЧ ЁЃ
ЂкзАжУЂѓжаЕФЪдМСПЩвдЪЧ ЃЈбЁЬюађКХЃЌЯТЭЌЃЉЃЌЯжЯѓЪЧ ЃЌдђжЄУїЦјЬхВњЮяжаКЌгаSO2ЃЛ зАжУЂєжаЕФЪдМСПЩвдЪЧ ЁЃ
| AЃЎ2 mol/LNa2CO3ШмвК |
| BЃЎЦЗКьШмвК |
| CЃЎ0.5 mol/LBaCl2ШмвК |
| DЃЎ0.5 mol/LBa(NO3)2 |
| ВйзїВНжш | дЄЦкЪЕбщЯжЯѓ | дЄЦкЪЕбщНсТл |
| ЯђЦфжавЛЗнШмвКжаМгШы ЁЃ | | ЙЬЬхжаКЌгаFe2O3 |
| ЯђСэвЛЗнШмвКжаЕЮМг2ЕЮЛЦЩЋK3[Fe(CN)6]ШмвКЁЃ | ВњЩњРЖЩЋГСЕэ | |
ЃЈ1ЃЉ4ЃЈFeSO4ЁЄ7H2OЃЉ+ O2= 4Fe(OH)SO4 + 26H2O
ЃЈ2ЃЉЂй50ЁцЃЌЗРжЙВњЩњЕЙЮќЃЈЛђЁАгУзїАВШЋЦПЁБЃЉ
ЂкCЃЌ ВњЩњАзЩЋГСЕэЃЌ BЁЂE
Ђл SO2 + 2OHЁЅ=SO32ЁЅ+ H2O
ЂмВйзїВНжш дЄЦкЪЕбщЯжЯѓ дЄЦкЪЕбщНсТл KSCNШмвК(ЛђСђЧшЛЏМиШмвК) ШмвКБфГЩбЊКьЩЋ ЙЬЬхжаКЌгаFeO
Ђн35.7%
НтЮіЪдЬтЗжЮіЃКЃЈ1ЃЉFe2+гаЛЙдадЃЌШнвзБЛПеЦјжаЕФбѕЦјбѕЛЏЮЊFe3+. ИљОнЬтвтИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ4ЃЈFeSO4ЁЄ7H2OЃЉ+ O2= 4Fe(OH)SO4 + 26H2OЁЃЃЈ2ЃЉЂйЗДгІЕФВњЮяFe2O3СєдкЪдЙмжаЃЌЖјSO2ЁЂSO3дђвдЦјЬхЕФаЮЪНДгЪдЙмжаГіРДЃЌШєЂђзАжУЩеБжаЫЎЕФдк0ЁцЁЂ25ЁцЃЌSO3ОЭЛсБфЮЊЙЬЬхЕЅжЪЃЌЕМжТзАжУжаЕФЦјЬхбЙЧПБфаЁЃЌЖјв§Ц№ЕЙЮќЯжЯѓЕФЗДгІЁЃвђДЫЂђзАжУЮТЖШгІПижЦдк50ЁцОЭПЩвдБЃжЄSO3ЕФЦјЬЌаЮЪНЁЃЂђзАжУЕФзїгУОЭЪЧЗРжЙВњЩњЕЙЮќЃЈЛђЁАгУзїАВШЋЦПЁБЃЉЁЃ ЂкЮоТлЪЧФФжжЗжНтЗНЪНЖМЛсВњЩњSO3ЃЌSO3ШмгкЫЎВњЩњСђЫсЁЃЫљвдзАжУЂѓжаЕФЪдМСПЩвдЪЧCЃЈBaCl2ЃЉЃЌЗДгІЕФРызгЗНГЬЪНЮЊЃКBa2++SO42-=BaSO4Ё§ЁЃЛсПДЕНВњЩњАзЩЋГСЕэЁЃШєвЊжЄУїЦјЬхВњЮяжаКЌгаSO2ЃЌПЩвдРћгУЦфЦЏАзадЃЌАбЦјЬхЭЈШыЦЗКьШмвКжаЃЌПДЕНЦЗКьШмвКЭЪЩЋЃЛЛђРћгУЦфЛЙдадЃЌАбЦјЬхЭЈШыЕНЫсадИпУЬЫсМиШмвКЁЃПДМћШмвКЕФзЯЩЋЭЪШЅЁЃвђДЫзАжУЂєжаЕФЪдМСПЩвдЪЧBЁЂEЁЃЂлSO2ЪЧДѓЦјЮлШОЮяЃЌдкХХЗХЕНПеЦјжЎЧАвЊНјааЮВЦјДІРэЁЃвђЮЊЫќЕФЫЎШмвКЯдЫсадЃЌЫљвдГЃгУМюШмвКРДЮќЪеДІРэЃЌШєзАжУVжаЪдМСЮЊNaOHШмвКЃЌЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊSO2 + 2OHЁЅ =SO32ЁЅ+ H2O ЁЃЂмШєЙЬЬхЪЧFe2O3ЃЌМгШыСђЫсЪБЗЂЩњЗДгІЃКFe2O3+3H2SO4= Fe2 (SO4) 3+3H2O.ВњЩњЕФFe3+гіЕНSCN-ШмвКЪБЛсЗЂЩњбеЩЋБфЛЏВњЩњбЊКьЩЋЕФFe(SCN)3. ШєЯђСэвЛЗнШмвКжаЕЮМг2ЕЮЛЦЩЋK3[Fe(CN)6]ШмвКЃЌВњЩњРЖЩЋГСЕэЁЃдђжЄУїКЌгаЙЬЬхжаКЌгаFeOЁЃЗДгІЕФРызгЗНГЬЪНЮЊFeO+4H+= Fe2++H2O. 3Fe2++2[Fe(CN)6]3-= Fe3[Fe(CN)6]2 Ё§. Fe3[Fe(CN)6]2ЪЧРЖЩЋФбШмадЕФЮяжЪЁЃЂнn (FeSO4)="22.8" gЁТ152 g/mol ="0.15mol.," МйЩшЫљЕУЙЬЬхжаFe2O3ЕФжЪСПЮЊx,FeOжЪСПЮЊy,дђx+y="11.2;" 2xЁТ160+ yЁТ72=0.15НтЕУx="4" ;y-7.2,ЫљвдFe2O3ЕФжЪСПЗжЪ§ЮЊ(4ЁТ11.2)ЁС100%=35.7%.
ПМЕуЃКПМВщFe2+ЕФаджЪЁЂРызгЗНГЬЪНЕФЪщаДЁЂЮВЦјЕФДІРэЁЂFe2+КЭFe3+ЁЂSO3КЭSO2ЕФМьбщЗНЗЈЁЂЪдМСЕФбЁдёгыЪЙгУМАЛьКЯЮяжаФГГЩЗжКЌСПЕФВтЖЈЕШжЊЪЖЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКМЦЫуЬт
ТЬЗЏ(FeSO4ЁЄ7H2O)СђЫсЗЈЩњВњвЛжжЯЁгаН№ЪєВњЦЗЙ§ГЬжаВњГіЕФИБВњЦЗЃЌВњЦЗЭтЙлЮЊЕТЬЩЋЛђЕЛЦТЬЩЋНсОЇЙЬЬхЁЃМгШыЪЪСППЩЕїНкМюадЫЎжаЕФpHЃЌгыЫЎжааќИЁЮягаЛњНсКЯЃЌВЂМгЫйГСЕэЃЌжївЊгІгУгкЫЎжЪОЛЛЏКЭЙЄвЕЗЯЫЎДІРэЃЌЭЌЪБОпгаЩБОњзїгУЁЃ
ЃЈ1ЃЉ98% 1.84 g/cm3ЕФХЈСђЫсдкЯЁЪЭЙ§ГЬжаЃЌУмЖШЯТНЕЃЌЕБЯЁЪЭжС50%ЪБЃЌУмЖШЮЊ1.4g/cm3ЃЌ50%ЕФСђЫсЮяжЪЕФСПХЈЖШЮЊ (БЃСєСНЮЛаЁЪ§)ЃЌ50%ЕФСђЫсгы30%ЕФСђЫсЕШЬхЛ§ЛьКЯЃЌЛьКЯЫсЕФХЈЖШЮЊ (Ью>ЁЂ<ЁЂ=")40%" ЁЃ
ЃЈ2ЃЉЪЕМЪЩњВњгУ20%ЗЂбЬСђЫс(100ПЫЗЂбЬСђЫсКЌSO3 20ПЫ)ХфжЦЯЁСђЫсЃЌШєгУSO3ЁЄnH2OБэЪО20%ЕФЗЂбЬСђЫсЃЌдђn=____________(БЃСєСНЮЛаЁЪ§)ЁЃ
ЃЈ3ЃЉТЬЗЏдкПеЦјжаШнвзБЛВПЗжбѕЛЏЮЊСђЫсЬњЃЌЯжШЁ7.32ПЫОЇЬхШмгкЯЁбЮЫсКѓЃЌМгШызуСПЕФBaCl2ШмвКЃЌЙ§ТЫЕУГСЕэ9.32ПЫЃЛдйЭЈШы112mL(БъзМзДПі)ТШЦјЧЁКУНЋFe2ЃЋЭъШЋбѕЛЏЃЌЭЦВтОЇЬхЕФЛЏбЇЪНЮЊ ЁЃ
ЃЈ4ЃЉСђЫсбЧЬњяЇЃл(NH4)2SO4ЁЄFeSO4ЁЄ6H2OЃн(ЫзГЦФЊЖћбЮ)ЃЌНЯТЬЗЏЮШЖЈЃЌдкЗжЮіЛЏбЇжаГЃгУРДХфжЦFe2+ЕФБъзМШмвКЃЌгУДЫFe2+ЕФБъзМШмвКПЩвдВтЖЈЪЃгрЯЁЯѕЫсЕФСПЁЃЯжШЁ8.64ПЫCu2SКЭCuSЕФЛьКЯЮягУ200 mL 2 mol/LЯЁЯѕЫсШмвКДІРэЃЌЗЂЩњЗДгІШчЯТЃК
10NO3-ЃЋ3Cu2SЃЋ16HЃЋ=6Cu2ЃЋЃЋ10NOЁќЃЋ3SO42-ЃЋ8H2O
8NO3-ЃЋ3CuSЃЋ8HЃЋ=3Cu2ЃЋЃЋ3 SO42-ЃЋ8NOЁќ+ 4H2O
ЪЃгрЕФЯЁЯѕЫсЧЁКУгыV mL 2 mol/L (NH4)2Fe(SO4)2ШмвКЭъШЋЗДгІЁЃ
вбжЊЃКNO3-ЃЋ3Fe2ЃЋЃЋ4HЃЋ= NOЁќЃЋ3Fe3+ЃЋ2H2O
Ђй VжЕЗЖЮЇ ЃЛ
Ђк ШєV=48ЃЌЪдМЦЫуЛьКЯЮяжаCuSЕФжЪСПЗжЪ§ (БЃСєСНЮЛаЁЪ§)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
гавЛКЌNaClЁЂNa2CO3ЁЄ10H2OКЭNaHCO3ЕФЛьКЯЮяЃЌФГЭЌбЇЩшМЦШчЯТЪЕбщЃЌ
ЭЈЙ§ВтСПЗДгІЧАКѓCЁЂDзАжУжЪСПЕФБфЛЏЃЌВтЖЈИУЛьКЯЮяжаИїзщЗжЕФжЪСПЗжЪ§ЁЃ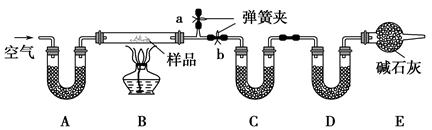
(1)МгШШЧАЭЈШыПеЦјЕФФПЕФЪЧ ЃЌ
ВйзїЗНЗЈЮЊ ЁЃ
(2)зАжУAЁЂCЁЂDжаЪЂЗХЕФЪдМСЗжБ№ЮЊЃКA ЃЌ
C ЃЌD ЁЃ
(3)ШєНЋAзАжУЛЛГЩЪЂЗХNaOHШмвКЕФЯДЦјЦПЃЌдђВтЕУЕФNaClЕФКЌСПНЋ (ЬюЁАЦЋИпЁБЁЂЁАЦЋЕЭЁБЛђЁАЮогАЯьЁБЃЌЯТЭЌ)ЃЛШєBжаЗДгІЙмгвВргаЫЎеєЦјРфФ§ЃЌдђВтЖЈНсЙћжаNaHCO3ЕФКЌСПНЋ ЃЛШєГЗШЅEзАжУЃЌдђВтЕУNa2CO3ЁЄ10H2OЕФКЌСПНЋ ЁЃ
(4)ШєбљЦЗжЪСПЮЊw gЃЌЗДгІКѓCЁЂDдіМгЕФжЪСПЗжБ№ЮЊm1 gЁЂm2 gЃЌгЩДЫПЩжЊЛьКЯЮяжаNaHCO3ЕФжЪСПЗжЪ§ЮЊ (гУКЌwЁЂm1ЁЂm2ЕФДњЪ§ЪНБэЪО)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
ФГЪЕбщаЁзщгћЭЈЙ§вдЯТЪЕбщРДЬНОПNa2CO3КЭNaHCO3СНжжЮяжЪЕФаджЪЁЃ
ЃЈ1ЃЉГЦШЁСНжжЙЬЬхИї2 gЃЌЗжБ№ЗХШыСНИіаЁЩеБжаЃЌдйИїЕЮМг10 mL еєСѓЫЎЃЌеёЕДЃЌВтСПЮТЖШБфЛЏЃЛД§ЙЬЬхГфЗжШмНтЃЌВЂЛжИДжСЪвЮТКѓЃЌЯђЫљЕУШмвКжаИїЕЮШы2ЕЮЗгЬЊЪдвКЁЃ
Ђй ЗЂЯжNa2CO3ЙЬЬхЭъШЋШмНтЃЌЖјNaHCO3ЙЬЬхгаЪЃгрЃЌгЩДЫЕУЕННсТл ЁЃ
Ђк ЭЌбЇУЧдкСНЩеБжаЛЙЙлВьЕНСЫЦфЫќЯжЯѓЁЃЦфжаЃЌЪЂЗХNa2CO3ЕФЩеБжаГіЯжЕФЯжЯѓЪЧ ЃЈЬюзжФИађКХЃЉЁЃ
AЃЎШмвКЮТЖШЯТНЕ BЃЎШмвКЮТЖШЩ§Ип CЃЎЕЮШыЗгЬЊКѓГЪЧГКьЩЋ DЃЎЕЮШыЗгЬЊКѓГЪКьЩЋ
ЃЈ2ЃЉШчЯТУцзѓЭМЫљЪОЗжБ№МгШШAЁЂBЙЬЬхЃЌЗЂЯжЙЬЬхAЪмШШВњЩњЕФЦјЬхФмЪЙГЮЧхЪЏЛвЫЎБфЛызЧЃЌЕЋвЛЖЮЪБМфКѓЛызЧгжБфГЮЧхЁЃЧыгУЛЏбЇЗНГЬЪННтЪЭГЮЧхЪЏЛвЫЎжаЗЂЩњЕФЯжЯѓ ЁЃ

ЃЈ3ЃЉШчЩЯЭМЫљЪОЃЌдкЦјУмадСМКУЕФзАжУIКЭIIжаЗжБ№ЗХШыЪдМСЃЌНЋЦјЧђФкЕФЙЬЬхЭЌЪБЕЙШыЪдЙмжаЁЃ
СНЪдЙмжаОљВњЩњЦјЬхЃЌ ЃЈЬюЁАIЁБЛђЁАIIЁБЃЉЕФЗДгІГЬЖШИќЮЊОчСвЁЃ
Ђк ЗДгІНсЪјКѓЃЌЦјЧђОљгаХђеЭЃЌЛжИДжСЪвЮТЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ ЁЃ
AЃЎзАжУIЕФЦјЧђЬхЛ§НЯДѓ BЃЎзАжУIIЕФЦјЧђЬхЛ§НЯДѓ
CЃЎЩњГЩЦјЬхЕФЬхЛ§ИљОнбЮЫсМЦЫу DЃЎЩњГЩЦјЬхЕФЬхЛ§ИљОнЙЬЬхМЦЫу
ЃЈ4ЃЉНЋСНжжЙЬЬхЗжБ№ХфжЦГЩ0.5 molЁЄL-1ЕФШмвКЃЌЬНОПгы0.5 molЁЄL-1CaCl2ШмвКЗДгІЕФЧщПі
| ЪЕбщЗНАИ | дЄВтЯжЯѓ | дЄВтвРОн | ЪЕМЪНсЙћ |
| ЪЕбщ1ЃКЯђ2 mL Na2CO3ШмвКжаЕЮМг1 mL 0.5 molЁЄL-1CaCl2ШмвК | гаАзЩЋ ГСЕэ | Na2CO3ШмвКжаЕФCO32-ХЈЖШНЯДѓЃЌФмгыCaCl2ЗЂЩњЗДгІЁЃ | гаАзЩЋГСЕэ |
| ЪЕбщ2ЃКЯђ2 mL NaHCO3ШмвКжаЕЮМг1 mL 0.5 molЁЄL-1CaCl2ШмвК | ЮоАзЩЋ ГСЕэ | NaHCO3ШмвКжаЕФCO32-ХЈЖШКмаЁЃЌВЛФмгыCaCl2ЗДгІЁЃ | гаАзЩЋГСЕэГіЯжЃЌЭЌЪБгаЩйСПЦјХнУАГіЁЃ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
ФГбаОПаЁзщРћгУШчЭМзАжУЬНОПЮТЖШЖдCOЛЙдFe2O3ЕФгАЯь(ЙЬЖЈзАжУТд)ЁЃ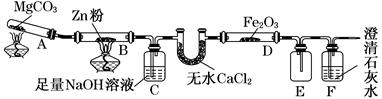
(1)MgCO3ЕФЗжНтВњЮяЮЊ________ЁЃ
(2)зАжУCЕФзїгУЪЧ_____________________________________________________ЃЌ
ДІРэЮВЦјЕФЗНЗЈЮЊ_______________________________________________________ЁЃ
(3)НЋбаОПаЁзщЗжЮЊСНзщЃЌАДШчЭМзАжУНјааЖдБШЪЕбщЃЌМззщгУОЦОЋЕЦЁЂввзщгУОЦОЋХчЕЦЖдзАжУDМгШШЃЌЗДгІВњЮяОљЮЊКкЩЋЗлФЉ(ДПОЛЮя)ЁЃСНзщЗжБ№гУВњЮяНјаавдЯТЪЕбщЃК
(вбжЊЃКFe2ЃЋгыK3[Fe(CN)6]ЗДгІВњЩњРЖЩЋГСЕэ)ЁЃ
| ВНжш | Вйзї | МззщЯжЯѓ | ввзщЯжЯѓ |
| 1 | ШЁКкЩЋЗлФЉМгШыЯЁбЮЫс | ШмНтЃЌЮоЦјХн | ШмНтЃЌгаЦјХн |
| 2 | ШЁВНжш1жаШмвКЃЌЕЮМгK3[Fe(CN)6]ШмвК | РЖЩЋГСЕэ | РЖЩЋГСЕэ |
| 3 | ШЁВНжш1жаШмвКЃЌЕЮМгKSCNШмвК | БфКь | ЮоЯжЯѓ |
| 4 | ЯђВНжш3ШмвКжаЕЮМгаТжЦТШЫЎ | КьЩЋЭЪШЅ | ЯШБфКьЃЌКѓЭЪЩЋ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
Fe2ЃЋКЭIЃЪЧСНжжГЃМћЕФЛЙдадРызгЁЃ
ЃЈ1ЃЉЯђFeSO4ШмвКжаЕЮМгТШЫЎЃЌШмвКгЩЧГТЬЩЋБфГЩЛЦЩЋЃЌЗДгІЕФРызгЗНГЬЪНЮЊ ЃЛЯђKIШмвКжаЕЮМгТШЫЎЃЌШмвКгЩЮоЩЋБфГЩЛЦЩЋЃЌЗДгІЕФРызгЗНГЬЪНЃК ЁЃ
ЃЈ2ЃЉЧывдFeSO4ШмвКЁЂKIШмвКЁЂТШЫЎЮЊЪдМСбщжЄIЃЕФЛЙдадЧПгкFe2ЃЋЁЃЩшМЦЪЕбщЗНАИЃЌВЙГфЭъГЩЪЕбщВНжшЁЂдЄЦкЯжЯѓКЭНсТлЁЃЦфЫћЯобЁЪдМСЃК3 molЁЄLЃ1 H2SO4ЁЂ0.01 molЁЄLЃ1 KMnO4ЁЂ20% KSCNЁЂ3%H2O2ЁЂЕэЗлШмвКЁЂзЯЩЋЪЏШяШмвКЁЃ
| ЪЕбщВНжш | дЄЦкЯжЯѓгыНсТл |
| ВНжш1ЃКШЁ2mLFeSO4ШмвККЭ2mLKIШмвКЛьКЯгкЪдЙмжаЃЌдйЕЮМг1ЁЋ2ЕЮТШЫЎЁЃ | ЃЛ |
| ВНжш2ЃК____________________________________ ____________________________________ЁЃ | |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
ЬњдкРфЕФХЈСђЫсжаФмЗЂЩњЖлЛЏЁЃФГаЫШЄаЁзщЕФЭЌбЇЗЂЯжНЋвЛЖЈСПЕФЬњгыХЈСђЫсМгШШЪБЃЌЙлВьЕНЬњЭъШЋШмНтЃЌВЂВњЩњДѓСПЦјЬхЁЃЪЕбщЪвЯжгаЯТСаЪдМСЃК0.01 mol/L ЫсадKMnO4ШмвКЁЂ0.1 mol/L KIШмвКЁЂ3ЃЅH2O2ШмвКЁЂЕэЗлШмвКЁЂеєСѓЫЎЁЃЧыФуажњЫћУЧЬНОПЫљЕУШмвККЭЦјЬхЕФГЩЗжЁЃ
ЁОЬсГіВТЯыЁП
Ђё.ЫљЕУШмвКжаЕФН№ЪєРызгПЩФмКЌгаFe2+КЭFe3+жаЕФвЛжжЛђСНжжЃЛ
Ђђ.ЫљЕУЦјЬхжаПЩФмКЌга_________жаЕФвЛжжЛђСНжжЁЃ
| | ЪЕбщВйзї | дЄЦкЯжЯѓ | НсТл |
| бщжЄВТЯыЂё | ВНжшЂйЃКШЁЩйСП0.01 mol/LЫсадKMnO4ШмвКЃЌЕЮШыЫљЕУШмвК | | |
| ВНжшЂкЃК_________ | | КЌгаFe3ЃЋ | |
| бщжЄВТЯыЂђ | НЋЫљЕУЦјЬхЭЈШыШчЯТзАжУ | | КЌгаСНжжЦјЬх |

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
ЬњПѓЪЏжївЊГЩЗжЮЊЬњЕФбѕЛЏЮяЃЈЩшдгжЪжаВЛКЌЬњдЊЫиКЭбѕдЊЫиЃЌЧвдгжЪВЛгыH2SO4ЗДгІЃЉЁЃФГбаОПадбЇЯАаЁзщЖдФГЬњПѓЪЏжаЬњЕФбѕЛЏЮяЕФЛЏбЇЪННјааЬНОПЁЃ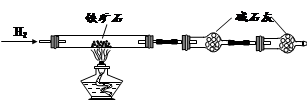
A B C
ЂёЃЎЬњПѓЪЏжаКЌбѕСПЕФВтЖЈ
Ђй АДЩЯЭМзщзАвЧЦїЃЌМьВщзАжУЕФЦјУмадЃЛ
Ђк НЋ5.0gЬњПѓЪЏЗХШыгВжЪВЃСЇЙмжаЃЌзАжУBЁЂCжаЕФвЉЦЗШчЭМЫљЪОЃЈМаГжвЧЦїОљЪЁТдЃЉЃЛ
Ђл ДгзѓЖЫЕМЦјЙмПкДІЛКЛКЭЈШыH2ЃЌ____________ЃЌЕуШМAДІОЦОЋЕЦ
Ђм ГфЗжЗДгІКѓЃЌГЗЕєОЦОЋЕЦЃЌдйГжајЭЈШыЧтЦјжСЭъШЋРфШДЁЃ
ЃЈ1ЃЉзАжУCЕФзїгУЮЊ________________________________________________ЁЃ
ЃЈ2ЃЉЂлжаЕуШМAДІОЦОЋЕЦЧАЫљашВйзїЮЊЁЁ______________________________ ЁЃ
ЃЈ3ЃЉВтЕУЗДгІКѓзАжУBдіжи1.35gЃЌдђЬњПѓЪЏжабѕЕФАйЗжКЌСПЮЊ____________ЁЃ
ЂђЃЎЬњПѓЪЏжаКЌЬњСПЕФВтЖЈ
ЃЈ1ЃЉВНжшЂмжажѓЗаЕФзїгУЪЧ__________________________________________ЁЃ
ЃЈ2ЃЉВНжшЂнжагУЕНЕФВЃСЇвЧЦїгаЩеБЁЂВЃСЇАєЁЂНКЭЗЕЮЙмЁЂ____________ЁЃ
ЃЈ3ЃЉЯТСагаЙиВНжшЂоЕФВйзїжаЫЕЗЈе§ШЗЕФЪЧ__________________ЁЃ
aЃЎвђЮЊЕтЫЎЮЊЛЦЩЋЃЌЫљвдЕЮЖЈЙ§ГЬжаВЛашМгжИЪОМС
bЃЎЕЮЖЈЙ§ГЬжаПЩРћгУЕэЗлШмвКзїЮЊжИЪОМС
cЃЎЕЮЖЈЙмгУеєСѓЫЎЯДЕгКѓПЩвджБНгзАвК
dЃЎзЖаЮЦПВЛашвЊгУД§ВтвКШѓЯД
eЃЎЕЮЖЈЙ§ГЬжаЃЌблОІзЂЪгЕЮЖЈЙмжавКУцБфЛЏ
fЃЎЕЮЖЈбеЩЋБфЛЏКѓЃЌ30sФкШмвКВЛЛжИДдРДЕФбеЩЋдйЖСЪ§
ЃЈ4ЃЉШєЕЮЖЈЙ§ГЬжаЯћКФ0.5000molЁЄL?1KIШмвК20.00mLЃЌдђЬњПѓЪЏжаЬњЕФАйЗжКЌСПЮЊ____________ЁЃ
ЂѓЃЎгЩЂёЁЂЂђПЩвдЭЦЫуГіИУЬњПѓЪЏжаЬњЕФбѕЛЏЮяЕФЛЏбЇЪНЮЊ ЁЃ
ШчКЮМьбщТЫвКAжаЪЧЗёКЌгаFe2+__________ЃЈЬюбЁЯюзжФИЃЉЁЃ
A.ЯШМгKSCNШмвКЃЌдйМгТШЫЎ B.МгNaOHШмвК C.МгK3[Fe(CN)6]
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
СђЫсбЧЬњОЇЬхЃЈFeSO4·7H2OЃЉдквНвЉЩЯзїВЙбЊМСЁЃФГПЮЭтаЁзщВтЖЈИУВЙбЊМСжаЬњдЊЫиЕФКЌСПЃЌВЂМьбщИУВЙбЊМСЪЧЗёБфжЪЁЃЪЕбщВНжшШчЯТЃК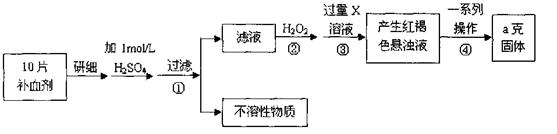
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉВНжшЂйТЫвКжаЕЮМгKSCNШмвККѓТЫвКБфЮЊКьЩЋЃЌдђИУШмвКжаКЌга (ЬюРызгЗћКХ)ЃЌМьбщТЫвКжаЛЙДцдкFe2+ЕФЗНЗЈЮЊ
ЃЈзЂУїЪдМСЁЂЯжЯѓЃЉЁЃ
ЃЈ2ЃЉВНжшЂкМгШыЙ§СПH2O2ЕФФПЕФЪЧ ЁЃ
ЃЈ3ЃЉВНжшЂлжаЗДгІЕФРызгЗНГЬЪНЮЊ ЁЃ
ЃЈ4ЃЉВНжшЂмжавЛЯЕСаДІРэЕФВйзїВНжшАќРЈЃКЙ§ТЫЁЂ ЁЂзЦЩеЁЂ ЁЂГЦСПЁЃ
ЃЈ5ЃЉШєЪЕбщЮоЫ№КФЃЌдђУПЦЌВЙбЊМСКЌЬњдЊЫиЕФжЪСПЮЊ gЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com