

ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D��

(1)CH3COOOH��Ϊ�������ᣬд������һ����;__________________________��
(2)д��B+E![]() CH3COOOH+H2O�Ļ�ѧ����ʽ__________________________����֪��ȩ����������Ҳ�����Ƶù������ᣬд���÷�Ӧ�Ļ�ѧ����ʽ______________________��
CH3COOOH+H2O�Ļ�ѧ����ʽ__________________________����֪��ȩ����������Ҳ�����Ƶù������ᣬд���÷�Ӧ�Ļ�ѧ����ʽ______________________��
(3)д��F���ܵĽṹ��ʽ______________________________��
(4)д��A�Ľṹ��ʽ_______________________________��
(5)1Ħ��C�ֱ�������Ľ���Na��NaOH��Ӧ������Na��NaOH���ʵ���֮����_________��
������(1)�����������ǿ�����ԣ�������ɱ��������
(2)�Ƚ�C2H4O2��![]() ����ɿ�֪B��E����ʱC��Hԭ�Ӹ�����û�����仯����ԭ�����࣬�ʲ���ˮ�е�H��Oԭ�ӱ���ԴE����������EΪH2O2��B+E
����ɿ�֪B��E����ʱC��Hԭ�Ӹ�����û�����仯����ԭ�����࣬�ʲ���ˮ�е�H��Oԭ�ӱ���ԴE����������EΪH2O2��B+E![]() CH3COOOH+H2O�Ļ�ѧ����ʽΪ
CH3COOOH+H2O�Ļ�ѧ����ʽΪ
![]()
(3)����C�Ľṹ�к��ǻ�����ŨH2SO4���������ȷ�����ȥ��Ӧ�ɵ� ���־����������Ȼ�֮����Ũ���������¿���ȥһ����ˮ����C
���־����������Ȼ�֮����Ũ���������¿���ȥһ����ˮ����C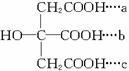 ��a��b��a��c�Ȼ�֮����ˮ�ɷֱ�õ���
��a��b��a��c�Ȼ�֮����ˮ�ɷֱ�õ���
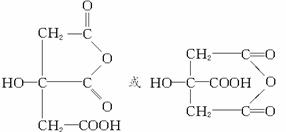
![]() ��֪DΪ��֧���ı���һԪ��CH3CH2CH2CH2��OH�����������Aˮ������B��C��D������B��C��D��ˮ�ɵ�A�Ľṹ��ʽ��
��֪DΪ��֧���ı���һԪ��CH3CH2CH2CH2��OH�����������Aˮ������B��C��D������B��C��D��ˮ�ɵ�A�Ľṹ��ʽ��
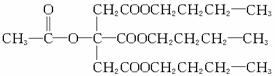
(5)1 mol C�к�1 mol �ǻ���3 mol �Ȼ����ǻ����Ȼ������Ʒ�Ӧ�������Ľ�����4 mol��ֻ���Ȼ���NaOH��Ӧ������3 mol NaOH���������ʵ���֮��Ϊ4��3��
�𰸣�(1)ɱ������


(5)4��3



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D��
��ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D��

(1)CH3COOOH��Ϊ�������ᣬд������һ����;________________________��
(2)д��B+E��CH3COOOH+H2O�Ļ�ѧ����ʽ______________________________________
(3)д��F���ܵĽṹ��ʽ_____________________________________________��
(4)д��A�Ľṹ��ʽ_____________________________________________��
(5)1Ħ��C�ֱ�������Ľ���Na��NaOH��Ӧ������Na��NaOH�����ʵ���֮����____��
(6)д��D��������(���廯�ƺ�Ũ����Ļ����)���ȷ�Ӧ�Ļ�ѧ����ʽ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D������ͼ����
��ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D������ͼ���� 
��1��CH3COOOH��Ϊ�������ᣬд������һ����;______________________________��
��2��д��B+E��CH3COOOH+H2O�Ļ�ѧ����ʽ_____________________________��
��3��д��F���ܵĽṹ��ʽ________________________________��
��4��1Ħ��C�ֱ�������Ľ���Na��NaOH��Ӧ������Na��NaOH���ʵ���֮����__________��
��5��д��D�������ᣨ���廯�ƺ�ŨH2SO4�Ļ������ȷ�Ӧ�Ļ�ѧ����ʽ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
( 12�� ) ��֪�����Ȼ�֮����Ũ������������ȥһ����ˮ������������:

ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D��

( 1 ) CH3COOOH��Ϊ�������ᣬд������һ����; _________ ��
( 2 ) д�� B + E ![]() CH3COOOH + H2O �Ļ�ѧ����ʽ ____________________ ��
CH3COOOH + H2O �Ļ�ѧ����ʽ ____________________ ��
( 3 ) д��F���ܵĽṹ��ʽ __________________ ��
( 4 ) д��A�Ľṹ��ʽ ______________________ ��
( 5 ) 1 mol C�ֱ�������Ľ���Na��NaOH��Ӧ������Na��NaOH���ʵ���֮���� _____ ��
( 6 ) д��D�������� ( ���廯�ƺ�Ũ����Ļ���� ) ���ȷ�Ӧ�Ļ�ѧ����ʽ:
______________________________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���ս̰���л�ѧѡ��5 4.3ȩ��������ϰ���������棩 ���ͣ������
��֪�����Ȼ�֮����Ũ������������ȥһ����ˮ�����������磺

ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D��

(1)CH3COOOH��Ϊ�������ᣬд������һ����;_______________��
(2)д��B+E��CH3COOOH+H2O�Ļ�ѧ����ʽ____________________��
(3)д��F���ܵĽṹ��ʽ____________________��
(4)д��A�Ľṹ��ʽ________________________��
(5)1 mol C�ֱ�������Ľ���Na\,NaOH��Ӧ������Na��NaOH���ʵ���֮����_______��
(6)д��D��������(���廯�ƺ�Ũ����Ļ����)���ȷ�Ӧ�Ļ�ѧ����ʽ��____________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com