
| Ӧ����NaOH������/g | �Ѹ����� | ���Ѹ��������Ҫ���������� |
| �ձ���ҩ�ס� ������ƽ |
| n |
| V |
| m |
| �� |
| 901.6 |
| 98% |
| m |
| �� |
| 920g |
| 1.84g/cm3 |
| n |
| V |


 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Һ | B������Һ |
| C������Һ | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ҹ��ڷ��˷ܼ������ϵ������Ǽ��֧�֡����İ��ˡ��ģ�ij���˷ܼ��Ľṹ��ͼ��ʾ����������������ȷ���ǣ�������
�ҹ��ڷ��˷ܼ������ϵ������Ǽ��֧�֡����İ��ˡ��ģ�ij���˷ܼ��Ľṹ��ͼ��ʾ����������������ȷ���ǣ�������| A�����ķ���ʽΪC20H22O3 |
| B����������ˮ |
| C�����ķ����й�ƽ���̼ԭ�������16�� |
| D��1mol��������7molH2�ͺ�4mol Br2����ˮ������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
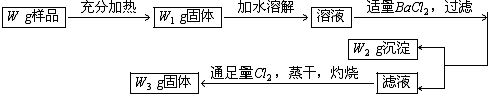
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
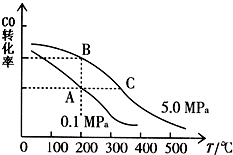 ��1����֪��C��s��+O2��g��=CO2��g����H1=-393.5kJ/mol
��1����֪��C��s��+O2��g��=CO2��g����H1=-393.5kJ/mol�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ɫƿ�� |
| B������CCl4�� |
| C������ˮ�� |
| D������ú���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������ƽ��ȡ11.7 g CuO��ĩ |
| B���ù㷺��pH��ֽ�����Һ��pHΪ6.2 |
| C���¶ȼ���ʾ�����¶���Ϊ25.69�� |
| D����100 mL��Ͳ��ȡ5.6 mL��ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����C��HCO3-��=0.1mol/L����Һ��NH4+��Al3+��Cl-��NO3- |
| B������ˮ�������C��H+��=1��10-12mol/L����Һ��AlO2-��HCO3-��Na+��SO42- |
| C����ʹ��ɫʯ����ֽ��������Һ��SO32-��CO32-��Na+��K+ |
| D��pH=1����Һ��Mg2+��Fe2+��NO3-��Cl- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com