
| ���������ܺ� |
| �������ʵ����ܺ� |
| m |
| V |
| ���������ܺ� |
| �������ʵ����ܺ� |
| 1-x+3-3x+2x |
| 4 |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ʼA�����ʵ���Ϊ2mol |
| B��5minʱB��ת����Ϊ16.67% |
| C��5minʱA��Ũ��Ϊ1.5 mol/L |
| D��x=3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
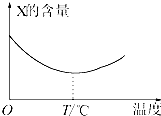 ������̬����X��Y��ֱ�ӻ�������Z�����淴Ӧ�����罫X��Y��һ��������ϲ�ѹ���ܱ������У��ڲ�ͬ�¶��¾���һ��ʱ���Ӧ�������X�ĺ����仯��ͼ��ʾ���ش��������⣺
������̬����X��Y��ֱ�ӻ�������Z�����淴Ӧ�����罫X��Y��һ��������ϲ�ѹ���ܱ������У��ڲ�ͬ�¶��¾���һ��ʱ���Ӧ�������X�ĺ����仯��ͼ��ʾ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
| A��-260.4 kJ?mol-1 |
| B��-441.8 kJ?mol-1 |
| C��260.4 kJ?mol-1 |
| D��441.8 kJ?mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ø������ֽ��ƶ���걾 |
| B����պ����������Ϊ75%�ľƾ������Ƥ�� |
| C���������ߵƹ����䲡�� |
| D��������Һ�мӱ���ʳ��ˮ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������Һ�У�Ba2+��K+��SO42-��Cl- |
| B�����д���Al3+����Һ�У�K+��Na+��NH4+��SO42- |
| C��ʹ���ȱ�Ƶ���Һ�У�Na+��I-��CO32-��OH- |
| D����ɫ��Һ�У�K+��Cl-��Cr2O72-��HCO3- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com