
| m��18% |
| 12 |
| m��10% |
| 1 |
| m��65% |
| 16 |
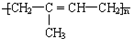 ����Ȼ�������Խṹǿ�Ⱥ����Բ��S�������ã�ʹ���ṹ����״�������״��������нϸߵ�ǿ�ȡ����Ժͻ�ѧ�ȶ��ԣ��ʴ�Ϊ��
����Ȼ�������Խṹǿ�Ⱥ����Բ��S�������ã�ʹ���ṹ����״�������״��������нϸߵ�ǿ�ȡ����Ժͻ�ѧ�ȶ��ԣ��ʴ�Ϊ��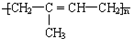 �� ��״�ṹ��
�� ��״�ṹ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������������Һ�����ᷴӦ |
| B��Ũ��������ˮ |
| C��þ��ϡ���ᷴӦ |
| D�����������������Ȼ�炙�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ������ | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 |
| pH����ʼ������ | 2.3 | 7.5 | 5.6 |
| pH����ȫ������ | 3.9 | 9.7 | 6.4 |
2- 3 |
2- 6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ˮ | B������� |
| C���Ȼ��� | D������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com