
ij����������ˮ��õ�����Һ�У�����Fe2+��Fe3+��SO42-��NH4+��Ba2+��CO32-�����е�ij���֡�
��1����ͬѧ��̽����Һ����ɣ�����������ʵ�飺��ȡ������Һ���Թ��У���μ���Ũ����������Һ�����ֿ�ʼ���ɰ�ɫ��������ɫ����Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ��ͬʱ���д̼�������ų�������ȡ������Һ���Թ��У��������������ữ���ٵμ��Ȼ�����Һ���а�ɫ�������ɡ�
����Һ��һ�����е������� ��
д�����а�ɫ����ת��Ϊ���ɫ�����Ļ�ѧ����ʽ ��
��2����ͬѧ��������ʵ�飺ȡ������Һ���Թ��У��μӼ������������Һ�������������ٵμ�H2O2��������Һ���ɫ�������μ�H2O2����ɫ����ȥ�������ݲ�����ΪŪ������Ե�ɣ���ͬѧ��������֪��
H2O2��SCN-��SO42-+CO2����N2����H2O��H+
�ٸ÷�Ӧ�У���������Ԫ��Ϊ ��ÿ����lmol CO2ת�Ƶĵ�����Ϊ NA��
�ڸ�����ͬѧ��ʵ���������жϻ�ԭ��ǿ��Ϊ��Fe2+ SCN-�����=����
�۸������ϣ���ͬѧ����IJ����ǣ�H2O2��SCN-����ʹ��ɫ����ȥ���������һ��ʵ�飬��֤��ͬѧ�IJ����Ƿ���ȷ ��
��12�֣�ÿ��2�֣�1.��1��Fe2+��SO42-��NH4+����1�ֿ�1�֣���д��д�����÷֣�
4Fe(OH)2+O2+2H2O��4Fe(OH)3 ��2����N��S 11 �� ��
��ȡ������ɫ�����Һ���Թ��У��μ�KSCN��Һ������Һ�ָ���ɫ��֤����ͬѧ�ƶ���ȷ
�������������1.��1��ȡ������Һ���Թ��У���μ���Ũ����������Һ�����ֿ�ʼ���ɰ�ɫ��������ɫ����Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ��ͬʱ���д̼�������ų�����˸���ʵ�������֪����ɫ���������������������ձ������������������������ԭ��Һ�к����������ӣ���һ��û��CO32-�������Ĵ̼�����ζ�������ǰ�����������Һ�л�����NH4+������ȡ������Һ���Թ��У��������������ữ���ٵμ��Ȼ�����Һ���а�ɫ�������ɣ��ð�ɫ����Ӧ�������ᱵ��������Һ�к���SO42-�����һ��û��Ba2+�����Ը���Һ��һ�����е�������Fe2+��SO42-��NH4+���������������ױ���������������������Ӧ�ķ���ʽΪ4Fe(OH)2+O2+2H2O��4Fe(OH)3��
��2���ٸ��ݷ�ӦH2O2��SCN-��SO42-+CO2����N2����H2O��H+��֪��˫��ˮ����Ԫ�صĻ��ϼ۴ӣ�1�۽��͵���2�ۣ��õ����ӣ�����ԭ��˫��ˮ����������SCN-��S��NԪ�صĻ��ϼ۷ֱ�ӣ�2�ۺͣ�3�����ߵ���6�ۺ�0�ۣ����Ա�������Ԫ����N��S��KSCN�ǻ�ԭ��������ÿ����lmol CO2ת�Ƶĵ��ӵ����ʵ�������6��2��0��3��mol��11mol����˵��Ӹ���Ϊ11NA��
��ȡ������Һ���Թ��У��μӼ������������Һ�������������ٵμ�H2O2��������Һ���ɫ����ʱ�������ӱ����������������ӣ���������KSCN��Һ��Ӧ�Ժ�ɫ�������μ�H2O2����ɫ����ȥ�������ݲ�������˵����ʱKSCN��Һ�����������Ի�ԭ��ǿ��Ϊ��Fe2+��SCN-��
�������H2O2��SCN-����ʹ��ɫ����ȥ����ֻ��Ҫȡ������ɫ�����Һ���Թ��У��μ�KSCN��Һ������Һ�ָ���ɫ������֤����ͬѧ�ƶ���ȷ��
���㣺�������Ӽ����빲����жϣ�������ԭ��Ӧ����ʽ����д�Լ��жϺͼ��㣻ʵ�鷽�������̽��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
| ������ | CO32����SiO32����AlO2����Cl�� |
| ������ | Al3����Cu2����Mg2����NH4+��Na�� |
 Sn(OH)2
Sn(OH)2 Sn2����2OH����
Sn2����2OH�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D�������ʾ�Ϊ����������ɵĿ����Ի����������������ʵ����ӣ����Ӳ����ظ���ϣ��У�
| ������ | Na+��Al3+��Ba2+��NH4+ |
| ������ | Cl����OH����CO32����SO42�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������������������ǿ�������������ɷ�����H1N1���С�
��1����̼������һ���ж�����;��������ϵ��̬Ư������ѧʽ�ɱ�ʾΪNa2CO3��3H2O2��������Na2CO3��H2O2��˫�����ʡ�
��H2O2��ʱ����Ϊ��ҵ��Һ���������������ɿ�ҵ��Һ�е��軯��(��NaCN)�������·�Ӧʵ�֣�NaCN��H2O2��H2O=A��NH3������������A�Ļ�ѧʽ______________
��ijǿ���Է�Ӧ��ϵ�У���Ӧ��������ﹲ�������ʣ�
O2��MnO4-��H2O��Mn2����H2O2��H������֪�÷�Ӧ��H2O2ֻ���������¹��̣�H2O2�� O2��
д���÷�Ӧ�����ӷ���ʽ��_______________________________________________��
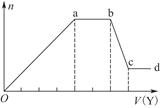
��2��ij��Ȼ��Ļ�ѧʽ�ɱ�ʾΪ:aNa2CO3��bNaHCO3��2H2O��ȡm g��Ȼ������ˮ�����Һ��������Һ����μ���1 mol/L�����ᣬ��״���²�����CO2������������������֮��Ĺ�ϵijͬѧ��������ͼ��ʾ��A��B���ߣ��Իش��������⣺
��_______������ȷ����Ȼ��Ļ�ѧʽΪ___________��
�ڼ���������CO2�������(��״��)�����ֵΪ _____________mL��
��3�� ����������ȱλ����пZnFe2Oy��������NOx��Ⱦ��ʹNOxת��ΪN2��ͬʱZnFe2Oyת��ΪZnFe2O4����2 mol ZnFe2Oy������NO2������0.5 mol N2����y��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о�С���û�����(FeS2)�������ƺ�������Һ��Ϸ�Ӧ�Ʊ�ClO2���壬����ˮ���ո�����ɵ�ClO2��Һ���ڴ˹�������Ҫ�������˵��¶ȣ����¶Ȳ���������Ӧ���ӣ�Ӱ������ClO2����Ĵ��ȣ��һ�Ӱ��ClO2�������ʣ����������ͼ��ʾ��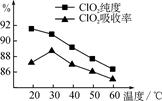
��1�� ��ͼ��֪����Ӧʱ��Ҫ���Ƶ������¶���________�棬Ҫ�ﵽ��Ҫ����Ҫ��ȡ�Ĵ�ʩ��______________��
��2�� ��֪���������е���Ԫ�������������¿ɱ�ClO3-������SO42-����д��FeS2�������ƺ�������Һ��Ϸ�Ӧ���ɶ�������(ClO2)�����ӷ���ʽ��______________________��
��3�� ��С�����ԡ�m(ClO2)/m(NaClO3)����Ϊ����ClO2���ʵ�ָ�ꡣ��ȡNaClO3��Ʒ6.0 g��ͨ����Ӧ�����ջ��400 mL ClO2��Һ��ȡ����Һ20 mL��37.00 mL 0.500 mol��L��1 (NH4)2Fe(SO4)2��Һ��ַ�Ӧ������Fe2������0.050 0 mol��L��1 K2Cr2O7����Һ�ζ����յ㣬����K2Cr2O7����Һ20.00 mL����Ӧԭ��Ϊ��
4H����ClO2��5Fe2��=Cl����5Fe3����2H2O
14H����Cr2O72-��6Fe2��=2Cr3����6Fe3����7H2O
�Լ���ClO2�ġ����ʡ�(д���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Һ�������������е�5�֣�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�Ҹ����������ʵ�����Ϊ1 mol ��
�����ӣ�Na+ ��Mg2+ ��Fe3+ ��Al3+ ��Fe2+ �������ӣ�OH����CO32����Cl����NO3����SO42����
��������Һ�м���KSCN��Һ�����Ա仯��
������ԭ��Һ�м���ϡ���ᣬ����ɫ�������ɣ���Һ������������䣻 ���ƶϣ�
��1��ԭ��Һ�к��������� ����������
��2����ԭ��Һ�м�������ϡ���ᷢ����Ӧ�����ӷ���ʽ
��3������ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ����ˣ�ϴ�ӣ����������أ��õ��Ĺ���
����Ϊ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Т���Ƭ ��NaCl��Һ �۰�ˮ �ܴ��� �ݾƾ� ������ ��H2SO4
��KOH���� ������ ��KAl(SO4)2��12H2O�������ܵ������ �����ڵ���ʵ���____ ___�����ڷǵ���ʵ��� ����������� �����ڼ���� ��������ţ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
KMnO4��һ����Ҫ����������
��1����������������KMnO4�������Ի���ǿ�������ữKMnO4��Һ������Լ��� ��
a������ b������ c������
�ڽ�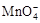 ����C2O42?�����ӷ���ʽ����������
����C2O42?�����ӷ���ʽ����������
��MnO4? +��C2O42?+�� ==��Mn2+ +��CO2��+�� ��
��2��ijͬѧΪ��̽��KMnO4��Һ��Na2C2O4�������ƣ���Һ�ķ�Ӧ���̣���������ʵ�飺
������100 mL 0.0400 mol��L-1��Na2C2O4��Һ�����õ�������ƽ��ҩ�ס��ձ�����Ͳ���������������⣬�������õ��IJ��������� ��
�ڽ�KMnO4��Һ��ε���һ�����������Na2C2O4��Һ�У��¶���ͬ��������������¼���������£�
| ����KMnO4��Һ�Ĵ��� | KMnO4��Һ��ɫ��ȥ�����ʱ�� |
| �ȵ����1�� | 60 s |
| ��ɫ���ٵ����2�� | 15 s |
| ��ɫ���ٵ����3�� | 3 s |
| ��ɫ���ٵ����4�� | 1 s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ѧУ�����ĺ�ˮ�и�Ƽ�賤������ˮ�ʶ���ˮˮ���п��ܺ���Fe3����Ba2����K����H+��NO3����Cl-��CO32-��SO42-���ӡ�Ϊ�˽�һ��ȷ�ϣ�ȡ������ʵ���⣺
��ȡˮ����ϸ�۲죬��������һ״̬��
����pH��ֽ�ⶨ��ˮ��pH����ֽ�Ժ�ɫ��
����ˮ���е���KSCN��Һ���ʺ�ɫ��
����ˮ���е���ϡ���ᣬ�д�����ɫ�����������ټ�ϡ���ᣬ��ɫ��������ʧ��
��1���ɴ˿�֪������ˮ�п϶����е�������_________���϶�û�е�������_________��
��2�� ��Ƽ�賤�Ŀ���ԭ����ˮ�к��н϶��_____________���ӡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com