
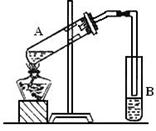
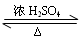



 Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ;�� | ����1 mol Al(OH)3����H+��OH�������ʵ���/mol | |
| ����H+ | ����OH�� | |
| 1��Al��Al3+��Al(OH)3 | | |
2��Al��AlO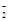 ��Al(OH)3 ��Al(OH)3 | | |
3��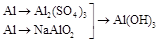 | | |
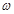 ��g��NaOH���塣����������ƽ�������Ϸ��루
��g��NaOH���塣����������ƽ�������Ϸ��루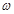 + y��(g)���룬�����̵ı������м���NaOH���壬��ʱָ��ƫ���ұߣ���ͼ��ʾ���������IJ���Ӧ����_________ ʹ ��
+ y��(g)���룬�����̵ı������м���NaOH���壬��ʱָ��ƫ���ұߣ���ͼ��ʾ���������IJ���Ӧ����_________ ʹ ��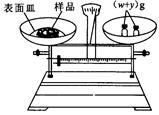
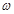 (g)NaOH�պÿ�����0.5mol��L��1NaOH��Һ500mL��������������Һ����ʾ��ͼ���д�����ǣ��������ţ�______________��
(g)NaOH�պÿ�����0.5mol��L��1NaOH��Һ500mL��������������Һ����ʾ��ͼ���д�����ǣ��������ţ�______________��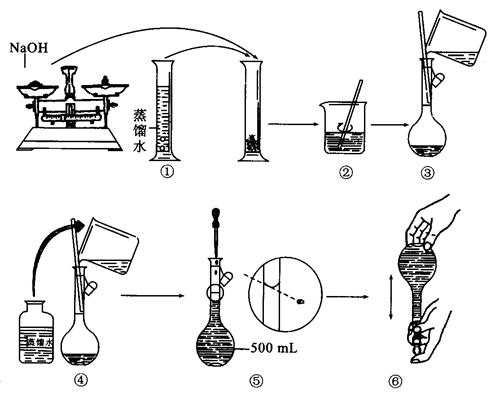
 NaOH��Һ���ټ���������м������Һ�Լ��ȡ��������ǣ�__________________________������ˮ����м��ϴ�ɾ���������м������Ϊm1(g)��
NaOH��Һ���ټ���������м������Һ�Լ��ȡ��������ǣ�__________________________������ˮ����м��ϴ�ɾ���������м������Ϊm1(g)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ũ��ˮ����CaO������ | B��NH4Cl��Һ��NaOHϡ��Һ��� |
| C��NH4Cl������ȷֽ� | D��������ʯ�Һ�NH4Cl�Ĺ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
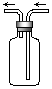
| A��NO | B��N2 | C��CO | D��SO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
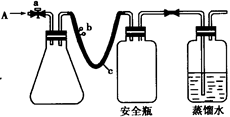
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
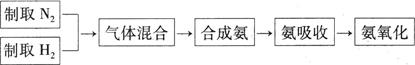
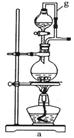

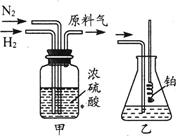
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com