
�����������еĻ��ţ�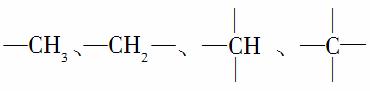 �е�̼ԭ�ӷֱ��Ϊ�����١��塢��̼ԭ�ӣ���Ŀ�ֱ���N1��N2��N3��N4��ʾ�����磺
�е�̼ԭ�ӷֱ��Ϊ�����١��塢��̼ԭ�ӣ���Ŀ�ֱ���N1��N2��N3��N4��ʾ�����磺
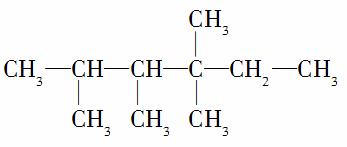
�����У�N1��6��N2��1��N3��2��N4��1���Ը��ݲ�ͬ��������ɽṹ������������(��������)�и�ԭ�����Ĺ�ϵ��
(1)������������ԭ����N0��N1��N2��N3��N4֮��Ĺ�ϵ��N0��________________________________________________________��
(2)4��̼ԭ����֮��Ĺ�ϵΪN1��_______________________��
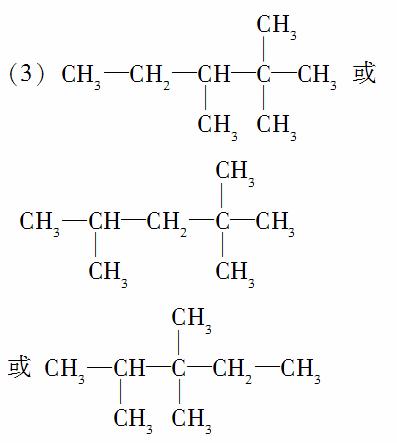
(3)��������N2��N3��N4��1����÷��ӵĽṹ��ʽ����Ϊ(��дһ��)________________________________��
������(1)����������ͨʽCnH2n��2��֪��N0��2(N1��N2��N3��N4)��2������ݽṹ��ʽ�Ƴ�N0��3N1��2N2��N3��
(2)ͨ�������ṹ��ʽ��֪���м��Cԭ��(ĩ�˵�2��—CH3����ټ���)�Ĺ�ϵΪ��һ��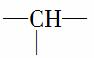 ��Ӧһ��—CH3��һ��
��Ӧһ��—CH3��һ��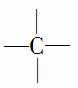 ��Ӧ2��—CH3��—CH2—���ܽ��—CH3(ĩ�˵�—CH3����)������N1��N3��2N4��2��
��Ӧ2��—CH3��—CH2—���ܽ��—CH3(ĩ�˵�—CH3����)������N1��N3��2N4��2��
(3)���������(2)���õĽ�������N1��1��2��1��2��5��
�𰸡�(1)2(N1��N2��N3��N4)��2��3N1��2N2��N3
(2)N3��2N4��2


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ȤС������50g��������Ϊ5%��NaCl��Һ����ش��������⣺
�Ų����ǣ��ټ��㡢�� �� �Ȼ��ơ��� �� ˮ ���� �� ����ת�ơ�
��ʵ���в������������ǣ� �� ��
����������Һ�Ĺ����У��ᵼ����Һ���Ȼ��Ƶ���������С��15%��ԭ������� �� ��
A������Ͳ��ȡˮʱ���Ӷ���
B�������Ȼ��ƹ���ʱ������ƽ������������
C���ܽ�ʳ�ε��ձ��ڱ��dz�ʪ��
D���������õ��Ȼ��ƹ��嵹���ձ���ʱ���в����Ȼ��ƹ���ɢ����ʵ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C��D��E��������
��A��������14�����ӣ�����M���Ӳ�����2������
��B���õ�2�����Ӻ�����Ӳ�ṹ��Ne��ͬ
��C������һ����λ������ɣ��˵����Ϊ11
��D��������18�����ӣ���ʧȥ1������ʱ�ʵ�����
��E�������磬��������Ϊ1
����д��A��B��C��D��E�����ķ��ţ�________��
________��________��________��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ����ȡ����Ӧ����(����)
A��C2H4��3O2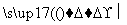 2CO2��2H2O
2CO2��2H2O
B��Zn��CuSO4===ZnSO4��Cu
C��NaCl��AgNO3===AgCl����NaNO3
D��CH2Cl2��Cl2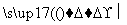 CHCl3��HCl
CHCl3��HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ʵ�У���֤�������������̼ԭ��Ϊ���ĵ���������ṹ����(����)
A��CH3Clֻ����һ������
B��CHCl3ֻ����һ������
C��CH2Cl2ֻ����һ������
D��CCl4ֻ����һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֤���Ҵ���������һ���ǻ�����ʵ��(����)
A���Ҵ���ȫȼ������CO2��H2O
B. 0.1 mol�Ҵ��������Ʒ�Ӧ����0.05 mol����
C. �Ҵ�����ˮ������Ȼ���
D. �Ҵ��ӷ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�Ҵ���ˮ���Ʒ�Ӧ������ֱ���________________________________________________________________________________��
�䷴Ӧ����ʽ�ֱ���________________________________________________________________________________________________________________________________________________��
(2)�Ҵ��ڿ�����ȼ�յ�����Ϊ________________________________________________________________________��
�䷴Ӧ����ʽΪ________________________________________________________________________��
(3)�Ҵ��������Ļ�ѧ����ʽΪ________________________________________________________________________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D���Ƕ�����Ԫ�أ�ԭ�Ӱ뾶D>C>A>B������A��B����ͬһ���ڣ�A��C����ͬһ���塣Cԭ�Ӻ�������������A��B��ԭ�Ӻ���������֮�ͣ�Cԭ��������ϵĵ�������Dԭ��������������4�����Իش�
(1)������Ԫ�طֱ��ǣ�A________��B________��
C________��D________��
(2)������Ԫ�����ڳ��³�ѹ�µ�Һ̬����̬�⻯����ȶ����ɴ�С��˳����__________________________________________��
(3)AԪ��ij��������DԪ��ij�����ﷴӦ���ɵ��ʵĻ�ѧ����ʽ��__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ס�����λͬѧ��ͭ��������ԭ�ϣ������������ȡ����ͭ�ķ�����
�����٣�ͭ��Ũ�������ֱ�ӷ�Ӧ,��Cu��CuSO4
�����ڣ���Cu��CuO��CuSO4,
��ش��������⣺
��1���������١���ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽΪ ��
�÷�Ӧ������Ũ����� �Ժ� �ԡ�
��2�������ַ���������Ϊ��һ�ַ���������? (����)��������
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com