
(1)������Ľṹ��ʽΪ__________________��
(2)���ڱ������ڳ���������ˮ��ͨ��ʳƷ�м��뱽�����ơ���֪��������ˮ��ҺDH��7��ԭ����(�û�ѧ����ʽ��ʾ)________________________________��
(3)�Ჴ������һ�ֱȱ��������ȫ��ʳƷ����������Ũ������������£����ǻ���������Ҵ���Ϸ�Ӧ�����Ჴ�������÷�Ӧ�Ļ�ѧ����ʽ��_______________________________��
(4)A���Ჴ������һ��ͬ���칹�塣��֪��
��A�������Ƶ�Cu(OH)2��Һ��Ӧ(����)��������ɫ�����������л���B��
��1 mol A�������1 mol NaOH��Ӧ��
��A���ӵı��������������ڵ�ȡ����������̼ԭ�ӹ��ɵIJ�����֧����
A�Ľṹ��ʽ��______________________________________________________��
(5)�л���B��һ���������ܷ�����ȥ��Ӧ���ɹ�Ƥ�ᣬ��ѧ����ʽΪ��_________________________________________��
(6)��Ƥ����һ�������¿������㶹��(C9H6O2)���÷�Ӧ��������______________���㶹�صĽṹ��ʽΪ_________________________________��
��������(1)������Ľṹ��ʽΪ ![]()
(2)��������ˮ�ⷴӦ�Ļ�ѧ����ʽΪ ![]() ������дʱһ��Ҫд������š�
������дʱһ��Ҫд������š�![]() ��������д�ɡ�
��������д�ɡ�![]() ����
����
(3)���ǻ�������Ľṹ��ʽΪ![]() �������Ҵ�����������Ӧ�Ļ�ѧ����ʽΪ
�������Ҵ�����������Ӧ�Ļ�ѧ����ʽΪ![]()
![]() ������дʱ����Ҫ���Ƿ�Ӧ������Ũ���ᡢ���ȡ��Լ��������е�С����(H2O)��
������дʱ����Ҫ���Ƿ�Ӧ������Ũ���ᡢ���ȡ��Լ��������е�С����(H2O)��
(4)��С��ʵ���Ͼ���д������(4)�и�����3��������![]() ��ͬ���칹�塣A�Ľṹ��ʽ������
��ͬ���칹�塣A�Ľṹ��ʽ������![]() ��
��![]() ��
��
(5)B�Ľṹ��ʽ������ ��
��![]() ��B������ȥ��Ӧ�ķ���ʽΪ
��B������ȥ��Ӧ�ķ���ʽΪ
![]() (��
(��
![]()
(6)��Ƥ��Ľṹ��ʽΪ![]() �����к��еĹ�����Ϊ�ǻ����Ȼ�������֮����Է��������ڵ�������Ӧ�����㶹�أ������㶹�صĽṹ��ʽΪ
�����к��еĹ�����Ϊ�ǻ����Ȼ�������֮����Է��������ڵ�������Ӧ�����㶹�أ������㶹�صĽṹ��ʽΪ![]() ��
��
���𰸡���1��![]()





| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
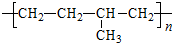 ���������Ժö����������������Ŵ��ܷ⽺������ҵ�����ұ��ĵ�����
���������Ժö����������������Ŵ��ܷ⽺������ҵ�����ұ��ĵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����������2007��4��ģ�⿼�Ի�ѧ���� ���ͣ�022
| |||||||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.��ʯȼ���ǿ������ģ���˵����ϵ��̲���Ҳ������
B.��ʯȼ����Ȼ�ڵ����ϵ��̲������ޣ����γɻ�ʯȼ�ϵ������൱�죬���Ի�ʯȼ���൱��������
C.��ʯȼ�ϵ��γ��Ƿdz����ӵģ�����ʱ��Ҳ�ϳ�������ʯȼ���ڵ����ϵ��̲���������
D.��ʯȼ���ڵ����ϵ��̲��������ģ������ֶ��Ǿ�������������γɵķ�������Դ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ˮƽ����ߣ�ʳƷ��ȫ���ⱶ������ע�������ᣨ![]() ����һ�ֱȽϰ�ȫ��ʳƷ��������
����һ�ֱȽϰ�ȫ��ʳƷ��������
��1��������Ľṹ��ʽΪ��_____________
��2�����ڱ������ڳ���������ˮ��ͨ��ʳƷ�м��뱽�����ơ���֪��������ˮ��ҺpH>7��ԭ���ǣ��û�ѧ����ʽ��ʾ��
__________________________________________________________________________
��3���Ჴ������һ�ֱȱ��������ȫ��ʳƷ����������Ũ������������£����ǻ���������Ҵ���Ϸ�Ӧ�����Ჴ�������÷�Ӧ�Ļ�ѧ����ʽ��
__________________________________________________________________________
��4��A���Ჴ������һ��ͬ���칹�塣��֪��
��A�������Ƶ�![]() ��Һ��Ӧ�����ȣ���������ɫ�����������л���B��
��Һ��Ӧ�����ȣ���������ɫ�����������л���B��
��1mol A�������1mol NaOH��Ӧ��
��A���ӵı��������������ڵ�ȡ����������̼ԭ�ӹ��ɵIJ�����֧����
A�Ľṹ��ʽ�ǣ�__________________________
��5���л���B��һ���������ܷ�����ȥ��Ӧ���ɹ�Ƥ�ᣬ��ѧ����ʽΪ��
_____________________________________________
��6����Ƥ����һ�������¿������㶹�أ�![]() ����
����
�÷�Ӧ��������_______________________________________
�㶹�صĽṹ��ʽΪ��_______________________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com