
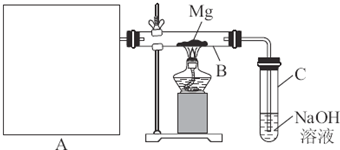
(1)���ݷ�ӦNa2SO3(s)+H2SO4(Ũ)====Na2SO4+SO2 ��+H2O���Ʊ�SO2���塣
�������м�ͼ���ڴ���ķ����л����Ʊ����ռ�SO2��ʵ��װ��(���Լ�)ʾ��ͼ��

ͼ6-34
��ʵ������У�ʹ�÷�Һ©���μ�Ũ����IJ����ǣ�__________________________��
(2)��SO2����ֱ�ͨ��������Һ�У�
��Ʒ����Һ��������_______________________________________��
����ˮ��������___________________________________________��
��������Һ��������_____________________________________��
(3)��һС����ʵ���з��֣�SO2����������������º���ʵ������ܲ����ԣ����ֲ��������������⡣�����Ʋ���ܵ�ԭ��˵����Ӧ����֤����(���Բ�����)��
��ԭ��________________����֤����_______________________________________��
��ԭ��________________����֤����________________________________________��
��ԭ��________________����֤����________________________________________��
�����������Զ�������Ϊ���壬����ʵ������ȡԭ����ʵ��װ�õ�ѡ����������ʹ�ò��������ʼ��鼰ʵ��������쳣����Ľ����л��ؽ����һ�𣬿���������Ʊ�������Ǩ��Ӧ��������ʵ������ķ�������������
(1)�����Ʊ���������ķ�Ӧԭ�������Ʊ��������������Լ���״̬����Ӧ����ȷ������ķ���װ�ã��ɶ������������(�ܶȡ�ˮ���ԡ����Ե�)ȷ�����ռ�װ�ú�����װ�ã��ڴ˻����ϻ����ռ����������ʵ��װ��ͼ��
(2)�����������Ư���ԣ���ʹƷ����Һ��ɫ������������л�ԭ�ԣ��ɱ���ˮ��������ʹ��ˮ��ɫ������������������ԣ����뻹ԭ��������Һ��Ӧ���ɵ�����ʹ��Һ����ǡ�
(3)�������֪��ȡ������������У��������������ʵ������ܲ����ԣ����������������⣬����ֻ���������Լ��йأ���Na2SO3���ʣ�������ΪNa2SO4��������Һ�岻��Ũ����ȡ�
�𰸣�(1)��(��ͼ6-35)

ͼ6-35
�ڴ�Һ©���ϿڵĻ�����������Һ©���������������μ�
(2)����Һ��ɫ ����Һ��ɫ ����dz��ɫ����(����Һ�����)
(3)��Na2SO3���� ȡ�����������Թ��У�����������ˮ�����Һ���ȵ�������ϡ���ᣬ�ٵ���BaCl2��Һ�а�ɫ�������ɣ���֤���ù���Na2SO3����
�ڲ���Ũ���� �ýྻ������պȡ����������Ϳ��ֽ����ڣ���֤������Һ����Ũ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
| ||
| ||


| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
����ᣨH2C2O4����Һ����μ������Ը��������Һʱ����Һ��ɫ����������졣����ԭ��ij�о���ѧϰС���ϻ�ѧ��Ӧԭ�������������裬�������һϵ��ʵ�����̽����
ʵ�飨1��������֧�Թ��зֱ����10mL��Ũ�ȵIJ�����Һ���ڢں��Թ��м������������̹��塣Ȼ������֧�Թ��зֱ����5��0.1 mol��L-1�������ữ���������Һ���������£���1��
|
| �ٺ��Թ� | �ں��Թ� |
| ����ҩƷ | ������Һ �����ữ���������Һ | ������Һ �����ữ���������Һ �����̹��� |
| ʵ��������ɫʱ�䣩 | ��Һ��ɫ������30s�� | ��Һ��ɫ�ܿ죨2s�� |
ʵ�飨2��������֧�Թ��зֱ����5 mL��Ũ�ȵIJ�����Һ���ڢں��Թ����ٵμ�10��ϡ���ᣬȻ�������5��0.1mol��L-1�ĸ��������Һ���������£���2��
| ��� | �ٺ��Թ� | �ں��Թ� |
| ��ɫʱ�� ���� | 100 s | 90 s |
| �������ݲ������ں��Թܵ���Һ��ɫ�Ȣٺ��Թܵ���Һ�죬������ɫ�仯���£��Ϻ�ɫ����ɫ���Ⱥ�ɫ����ɫ����ɫ����ɫ�� |
ʵ�飨3����ȡ3֧�Թֱܷ����5 mL��Ũ�Ȳ�����Һ��Ȼ���ڢ١��ڡ��ۺ��Թ������μ���10�Ρ�1 mL��2 mLϡ������Һ��������5��0.1 mol��L-1�ĸ��������Һ��Ȼ�������¶�Ϊ65���ˮԡ�м��ȡ��۲�����3����
| ��� | �ٺ��Թ� | �ں��Թ� | �ۺ��Թ� |
| ��ɫʱ��
ʵ������ | 80 s | 100 s | 120 s |
| �������ݲ������ٺ��Թܵ���Һ��ɫ�Ȣڡ��ۺ��Թܵ���Һ�죬������ɫ�仯���£��Ϻ�ɫ����ɫ���Ⱥ�ɫ����ɫ����ɫ����ɫ�� |
��1�� ���о���ѧϰС���ʵ��Ŀ���� ��
��2�����о���ѧϰС�����ʵ��ʱ���õķ����ǿ�ѧʵ���г��õ�һ�ַ������÷����� ����
��3��ʵ�飨1���ó��Ľ����� ��
��4����ʵ�飨2����ʵ�飨3���ó��Ľ����ǣ�д���㣩
_________________________,_____________________,_______________;
��5�����й�����д��������Һ�����Ը��������Һ��Ӧ�����ӷ���ʽ��
H2C2O4�� MnO4���� �� Mn2���� �� H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������人�����ص���ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
S2C12��һ�ֽ��ɫ�ӷ���Һ�壬����ǿ�ҵ���Ϣ�ԣ��ڹ�ҵ�����Ͽ���������Ϊ��ʵ���Һϳ�S2C12��ij��ѧ�о���ѧϰС�����������̽���� ���������ϡ��� �����������110�桫140������Ӧ�����ɵõ�S2C12���� S���۵�Ϊ112��8�桢�е�Ϊ444��6�棻S2C12���۵�Ϊ
���������ϡ��� �����������110�桫140������Ӧ�����ɵõ�S2C12���� S���۵�Ϊ112��8�桢�е�Ϊ444��6�棻S2C12���۵�Ϊ 76�桢�е�Ϊ138�档
76�桢�е�Ϊ138�档
�� S2C12+C12 2SCl2���� S2C12��ˮ������Ӧ������H2S��SO2��H2SO3��H2SO4�ȡ���ClO3-+5Cl-+6H
2SCl2���� S2C12��ˮ������Ӧ������H2S��SO2��H2SO3��H2SO4�ȡ���ClO3-+5Cl-+6H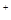 =3C12��+3H2O ��ش��������⣺
=3C12��+3H2O ��ش��������⣺ ��ʵ��װ����ơ�
��ʵ��װ����ơ�
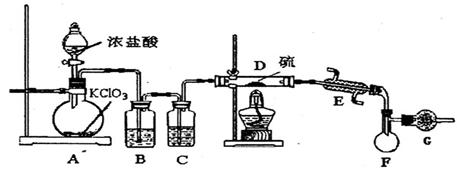

 ��1��B�������Լ�Ϊ �� C�������Լ�Ϊ ��
��1��B�������Լ�Ϊ �� C�������Լ�Ϊ �� ��2���ڼ���Dʱ�¶Ȳ��˹��ߣ���ԭ���� ��
��2���ڼ���Dʱ�¶Ȳ��˹��ߣ���ԭ���� ��
Ϊ�����S2C12�Ĵ��ȣ��ؼ��IJ����ǿ��ƺ��¶Ⱥ� �� ��3��Gװ�õ������� ��
��3��Gװ�õ������� ��
��4��д����ҵ����Ư�۵Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ�߶���ѧ�����п��Ի�ѧ�� ���ͣ������
����ᣨH2C2O4����Һ����μ������Ը��������Һʱ����Һ��ɫ����������졣����ԭ��ij�о���ѧϰС���ϻ�ѧ��Ӧԭ�������������裬�������һϵ��ʵ�����̽����
ʵ�飨1��������֧�Թ��зֱ����10mL��Ũ�ȵIJ�����Һ���ڢں��Թ��м������������̹��塣Ȼ������֧�Թ��зֱ����5��0.1 mol��L-1�������ữ���������Һ���������£���1��
|
|
�ٺ��Թ� |
�ں��Թ� |
|
����ҩƷ |
������Һ �����ữ���������Һ |
������Һ �����ữ���������Һ �����̹��� |
|
ʵ��������ɫʱ�䣩 |
��Һ��ɫ������30s�� |
��Һ��ɫ�ܿ죨2s�� |
ʵ�飨2��������֧�Թ��зֱ����5 mL��Ũ�ȵIJ�����Һ���ڢں��Թ����ٵμ�10��ϡ���ᣬȻ�������5��0.1 mol��L-1�ĸ��������Һ���������£���2��
|
��� |
�ٺ��Թ� |
�ں��Թ� |
|
��ɫʱ�� ���� |
100 s |
90 s |
|
�������ݲ������ں��Թܵ���Һ��ɫ�Ȣٺ��Թܵ���Һ�죬������ɫ�仯���£��Ϻ�ɫ����ɫ���Ⱥ�ɫ����ɫ����ɫ����ɫ�� |
ʵ�飨3����ȡ3֧�Թֱܷ����5 mL��Ũ�Ȳ�����Һ��Ȼ���ڢ١��ڡ��ۺ��Թ������μ���10�Ρ�1 mL��2 mLϡ������Һ��������5��0.1 mol��L-1�ĸ��������Һ��Ȼ�������¶�Ϊ65���ˮԡ�м��ȡ��۲�����3����
|
��� |
�ٺ��Թ� |
�ں��Թ� |
�ۺ��Թ� |
|
��ɫʱ��
ʵ������ |
80 s |
100 s |
120 s |
|
�������ݲ������ٺ��Թܵ���Һ��ɫ�Ȣڡ��ۺ��Թܵ���Һ�죬������ɫ�仯���£��Ϻ�ɫ����ɫ���Ⱥ�ɫ����ɫ����ɫ����ɫ�� |
��1�� ���о���ѧϰС���ʵ��Ŀ���� ��
��2�����о���ѧϰС�����ʵ��ʱ���õķ����ǿ�ѧʵ���г��õ�һ�ַ������÷����� ����
��3��ʵ�飨1���ó��Ľ����� ��
��4����ʵ�飨2����ʵ�飨3���ó��Ľ����ǣ�д���㣩
_________________________,_____________________,_______________;
��5�����й�����д��������Һ�����Ը��������Һ��Ӧ�����ӷ���ʽ��
H2C2O4�� MnO4���� �� Mn2���� �� H2O
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com