
(¢ń)ČēĶ¼ĖłŹ¾£¬¼×”¢ŅŅÖ®¼äµÄøō°åKŗĶ»īČūF¶¼æÉ×óÓŅŅĘ¶Æ£¬¼×ÖŠ³äČė2molAŗĶ1molB£¬ŅŅÖŠ³äČė2molCŗĶ1molHe£¬“ĖŹ±KĶ£ŌŚ0“¦£®ŌŚŅ»¶ØĢõ¼žĻĀ·¢ÉśæÉÄę·“Ó¦£ŗ
2A(g)£«B(g)![]() 2C(g)£»·“Ó¦“ļµ½Ę½ŗāŗó£¬ŌŁ»Öø“ÖĮŌĪĀ¶Č£®»Ų“šĻĀĮŠĪŹĢā£ŗ
2C(g)£»·“Ó¦“ļµ½Ę½ŗāŗó£¬ŌŁ»Öø“ÖĮŌĪĀ¶Č£®»Ų“šĻĀĮŠĪŹĢā£ŗ
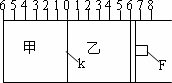
(1)æÉøł¾Ż________ĻÖĻóĄ“ÅŠ¶Ļ¼×”¢ŅŅ¶¼ŅŃ“ļµ½Ę½ŗā£®
(2)“ļµ½Ę½ŗāŹ±£¬øō°åK×īÖÕĶ£ĮōŌŚ0æĢ¶Č×ó²ąa“¦£¬ŌņaµÄȔֵ·¶Ī§ŹĒ________£®
(3)Čō“ļµ½Ę½ŗāŹ±£¬øō°åK×īÖÕĶ£ĮōŌŚ×ó²ąæĢ¶Č1“¦£¬Ōņ¼×ÖŠCµÄĪļÖŹµÄĮæĪŖ________mol£¬ŅŅÖŠCµÄ×Ŗ»ÆĀŹ________50£„(Ģī£ŗ£¾”¢£¼”¢£½)£¬“ĖŹ±£¬ŅŅÖŠæÉŅĘ¶Æ»īČūF×īÖÕĶ£ĮōŌŚÓŅ²ąæĢ¶Č________“¦(ĢīĻĀĮŠŠņŗÅ)¢Ł£¼6¢Ś£¾6¢Ū£½6£®
(4)Čō“ļµ½Ę½ŗāŹ±£¬øō°åK×īÖÕĶ£ĮōŌŚ×ó²ąæĢ¶Čææ½ü0“¦£¬ŌņŅŅÖŠæÉŅĘ¶Æ»īČūF×īÖÕĶ£ĮōŌŚÓŅ²ąµÄæĢ¶Č²»“óÓŚ________£»ČōK×īÖÕĶ£ĮōŌŚ×ó²ąæĢ¶Čææ½ü2“¦£¬ŌņŅŅÖŠF×īÖÕĶ£ĮōŌŚÓŅ²ąµÄæĢ¶Č²»Š”ÓŚ________£®
(¢ņ)ČōŅ»æŖŹ¼¾Ķ½«K”¢F¹Ģ¶Ø£¬ĘäĖüĢõ¼ž¾ł²»±ä£¬Ōņ“ļµ½Ę½ŗāŹ±£ŗ
(1)¼×”¢ŅŅÖŠCµÄĪļÖŹµÄĮæŹĒ________(Ģī£ŗ”°¼×£¾ŅŅ”±»ņ”°¼×£¼ŅŅ”±»ņ”°¼×£½ŅŅ”±)£»
(2)²āµĆ¼×ÖŠAµÄ×Ŗ»ÆĀŹĪŖb£¬ŌņŅŅÖŠCµÄ×Ŗ»ÆĀŹĪŖ________£»
(3)¼ŁÉčŅŅ”¢¼×Į½ČŻĘ÷ÖŠµÄŃ¹Ēæ±ČÓĆd±ķŹ¾£¬ŌņdµÄȔֵ·¶Ī§ŹĒ________£®

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ·Ö±š»Ų“šĻĀĮŠĪŹĢā£®
·Ö±š»Ų“šĻĀĮŠĪŹĢā£®| O | 2- 4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
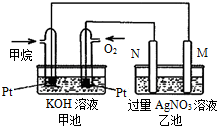
 ČēĶ¼ĖłŹ¾£¬¼×”¢ŅŅĮ½³Ųµē¼«²ÄĮĻ¶¼ŹĒĢś°ōŗĶĢ¼°ō£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ČēĶ¼ĖłŹ¾£¬¼×”¢ŅŅĮ½³Ųµē¼«²ÄĮĻ¶¼ŹĒĢś°ōŗĶĢ¼°ō£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ĢģČ»ĘųµÄÖ÷ŅŖ³É·Ö¼×ĶéČ¼ÉÕÉś³É¶žŃõ»ÆĢ¼ŗĶŅŗĢ¬Ė®µÄČČ»Æѧ·½³ĢŹ½ČēĻĀ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗCH4£Øg£©+2O2£Øg£©=CO2£Øg£©+2H2O£Øl£©”÷H=-889.6kJ/mol£®
ĢģČ»ĘųµÄÖ÷ŅŖ³É·Ö¼×ĶéČ¼ÉÕÉś³É¶žŃõ»ÆĢ¼ŗĶŅŗĢ¬Ė®µÄČČ»Æѧ·½³ĢŹ½ČēĻĀ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗCH4£Øg£©+2O2£Øg£©=CO2£Øg£©+2H2O£Øl£©”÷H=-889.6kJ/mol£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
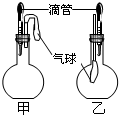 ČēĶ¼ĖłŹ¾µÄ¼×”¢ŅŅĮ½øö×°ÖĆÖŠ£¬½ŗĶ·µĪ¹ÜÖŠĪüČėijÖÖŅŗĢå£¬Ę½µ×ÉÕĘæÖŠ³äČė£Ø»ņ·ÅČė£©ĮķŅ»ÖÖĪļÖŹ£¬¼·Ń¹½ŗĶ·µĪ¹Ü¼ÓČėŅŗĢ壬Ņ»¶ĪŹ±¼äŗóĮ½×°ÖĆÖŠµÄĘųĒņ¶¼ÓŠĆ÷ĻŌµÄÕĶ“ó£ØŗöĀŌŅŗĢåĢå»ż¶ŌĘųĒņµÄÓ°Ļģ£©£®ŌņĖłÓĆŹŌ¼ĮæÉÄÜ·Ö±šŅĄ“ĪŹĒ£Ø””””£©
ČēĶ¼ĖłŹ¾µÄ¼×”¢ŅŅĮ½øö×°ÖĆÖŠ£¬½ŗĶ·µĪ¹ÜÖŠĪüČėijÖÖŅŗĢå£¬Ę½µ×ÉÕĘæÖŠ³äČė£Ø»ņ·ÅČė£©ĮķŅ»ÖÖĪļÖŹ£¬¼·Ń¹½ŗĶ·µĪ¹Ü¼ÓČėŅŗĢ壬Ņ»¶ĪŹ±¼äŗóĮ½×°ÖĆÖŠµÄĘųĒņ¶¼ÓŠĆ÷ĻŌµÄÕĶ“ó£ØŗöĀŌŅŗĢåĢå»ż¶ŌĘųĒņµÄÓ°Ļģ£©£®ŌņĖłÓĆŹŌ¼ĮæÉÄÜ·Ö±šŅĄ“ĪŹĒ£Ø””””£©| A”¢¼×£ŗÅØĮņĖįŗĶľĢæ ŅŅ£ŗÅØ°±Ė®ŗĶHBr | B”¢¼×£ŗĖ«ŃõĖ®ŗĶMnO2 ŅŅ£ŗNaOHČÜŅŗŗĶNO2 | C”¢¼×£ŗ±½·ÓŗĶNa2CO3ČÜŅŗ ŅŅ£ŗNaOHČÜŅŗŗĶCl2 | D”¢¼×£ŗÅØĮņĖįŗĶÕįĢĒ£ØµĪÓŠ¼øµĪĖ®£© ŅŅ£ŗĖ®ŗĶ°±Ęų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
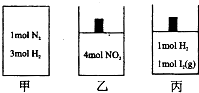 ČēĶ¼ĖłŹ¾µÄ¼×”¢ŅŅ”¢±ūČżøö¾ųČČČŻĘ÷ÖŠ·Ö±š·¢ÉśµÄ·“Ó¦ĪŖ£ŗ
ČēĶ¼ĖłŹ¾µÄ¼×”¢ŅŅ”¢±ūČżøö¾ųČČČŻĘ÷ÖŠ·Ö±š·¢ÉśµÄ·“Ó¦ĪŖ£ŗ| A”¢Čō¼×µÄĢå»żĪŖ2L£¬¾¹ż10Ćėŗó·“Ó¦“ļµ½Ę½ŗāדĢ¬£¬·Å³öČČĮæĪŖ55.44U£¬ŌņH2µÄ·“Ó¦ĖŁĀŹŹĒ0.09mol/£ØL?s£© | B”¢Čō¼×”¢ŅŅÖŠ·“Ó¦“ļµ½Ę½ŗāŹ±µÄĢå»żĻąĶ¬£¬ŌņĮ½ČŻĘ÷ÖŠĖłŗ¬ĪļÖŹµÄĮææÉÄÜĻąĶ¬ | C”¢ČōŅŅ”¢±ūÖŠ·“Ó¦“ļµ½Ę½ŗāŹ±µÄĢå»ż”¢Ń¹Ēæ¾łĻąĶ¬£¬ŌņŅŅÖŠNO2µÄ×Ŗ»ÆĀŹĪŖ50% | D”¢Čō¼×”¢ŅŅ”¢±ūÖŠ·“Ó¦¾ł“ļµ½Ę½ŗāדĢ¬Ź±£¬Ōņ¼×ÖŠĪļÖŹµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ²»±ä£¬ŅŅÖŠĪļÖŹµÄŃÕÉ«²»±ä£¬±ūÖŠµÄĪĀ¶Č²»±ä |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com