
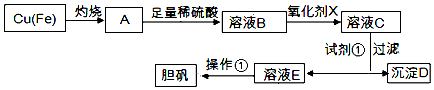
| ��Һ�б����������� | Fe3+ | Fe2+ | Cu2+ |
| ��ȫ������������ij���ʱ����Һ��pH | ��3.7 | ��6.4 | ��4.4 |
���� ���Ʊ�ʵ�����̿�֪��Fe��Cu���գ�ͭ��������Ӧ����CuO��Fe������������ȫ��Ӧ������ϡ���ᣬ��Ӧ��������ͭ��������������������Ϊ�˳�ȥ������������ȫ���������������ʱ��Һ��pH��֪���轫����������������������Ȼ�����pH�γɳ��������˳�ȥ����������������Һ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ɵõ������ݴ˽��
��� �⣺��1��������X�ǽ��������������������ӣ�ͬʱ���������µ����ʣ����Կ�����H2O2��
�ʴ�Ϊ��B��
��2�������Լ�����Ϊ�˵���pH������Һ�е������ӷ�Ӧ��ͬʱ���������µ����ʣ��Լ��ٿ���ѡ��CuO��CuCO3��Cu��OH��2��
�ʴ�Ϊ��CuO��CuCO3��Cu��OH��2��
��3���������Ǵ�����ͭ ��Һ�л������ͭ���壬������������Ũ������ȴ�ᾧ��
�ʴ�Ϊ������Ũ������ȴ�ᾧ��
��4��A����FeCl3������Һ��μ����ˮ�У����������ȵõ����ɫҺ��Ϊ�����������壬�������������ܲ��������ЧӦ����A��ȷ��
B����FeCl3��Һ�μ�NaOH��Һ�����������������ɫ��������B��ȷ��
C����FeCl3��Һ�������ɲ����գ������Ȼ���ˮ�⣬���յõ�Fe2O3���壬��C����
D����FeCl3��Һ�еμ�KSCN��Һ����Fe��SCN��3����Һ��Ϊ��ɫ����D��ȷ��
�ʴ�Ϊ��C��
��5��ʵ���ұ���FeCl2��Һ���������������۷�ֹFeCl2��Һ���ʣ������л�ԭ�ԣ����ӷ���ʽΪ��Fe+2Fe3+=3Fe2+��
�ʴ�Ϊ��Fe+2Fe3+=3Fe2+��
��6����̼�缫�������ͭ��Һ200mL��ͨ��5min����������0.64g������ΪΪͭ���ӷŵ��ͭ���ʣ�������Cu�����ʵ���Ϊ$\frac{0.64g}{64g/mol}$=0.01mol������ͭ�������ӵĹ�ϵʽCu��2H+֪�������ӵ����ʵ���Ϊ0.02mol����Һ��������Ũ��Ϊ$\frac{0.02mol}{0.1L}$=0.1mol/L������Һ��pH=lgc��H+��=1��
�ʴ�Ϊ��1��
���� ���⿼����ͭ���仯�������ʷ�������ѧ����ʽ���㣬�����Ʊ���ԭ���ͼ�������ݻ����ǹؼ�����Ŀ�Ѷ��еȣ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����25mL | B�� | ����25mL | C�� | С��25mL | D�� | ���ж� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
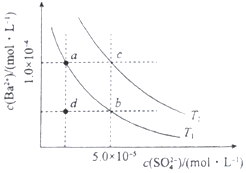 ���Ǽ���������ʵĵ���ƽ�ⳣ�������ܵ���ʵ��ܶȻ�Ksp��25�棩��
���Ǽ���������ʵĵ���ƽ�ⳣ�������ܵ���ʵ��ܶȻ�Ksp��25�棩��| ����� | ���뷽��ʽ | ���볣��K | Ksp |
| H2CO3 | H2CO3?HCO3-+H+ HCO3-?CO32-+H+ | K1=4.31��10-7 K2=5.61��10-11 | - |
| H3PO4 | H3PO4?H2PO4-+H+ H2PO4-?HPO42-+H+ HPO42-?PO43-+H+ | K1=7.52��10-3 K2=6.23��10-6 K3=2.20��10-13 | - |
| C6H5OH | C6H5OH?C6H5O-+H+ | 1.1��10-10 | - |
| NH3•H2O | NH3•H2O?OH-+NH4+ | 1.76��10-5 | - |
| BaSO4 | BaSO4��s��?Ba2++SO42- | - | 1.1��10-10 |
| BaCO3 | BaCO3��s��?Ba2++CO32- | - | 1��10-9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2H2 ��g��+O2��g��=2H2O��l�� | B�� | H2��g��+Cl2 ��g��=2HCl��g�� | ||
| C�� | ��NH4��2CO3��s��=NH4HCO3 ��s��+NH3��g�� | D�� | Cu��s��+Cl2 ��g��=CuCl2 ��s�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | -916.9 kJ/mol | B�� | -458.45 kJ/mol | C�� | +916.9 kJ/mol | D�� | +458.45 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������Ҫ�Ļ���ԭ�ϣ�ij̽��С���������з�Ӧ��ȡˮ���£�N2H4•H2O����
������Ҫ�Ļ���ԭ�ϣ�ij̽��С���������з�Ӧ��ȡˮ���£�N2H4•H2O�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��NaHCO3�������������ˮ�У�����ɫ���ݣ�H+�� | |
| B�� | ������ˮ�ʻ���ɫ��Cl2�� | |
| C�� | ʹ��ɫʯ����ֽ�ȱ�����ɫ��H+��Cl2�� | |
| D�� | �μ�AgNO3��Һ���ɰ�ɫ������Cl-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com