
�±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ��![]()
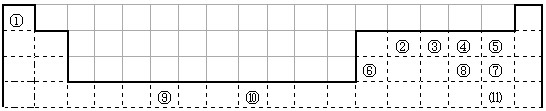
�����![]()
��1��д���١�����Ԫ�ذ�ԭ�Ӹ�����Ϊ1��1�γɵĻ�����ĵ���ʽ ��д���ϱ���Ԫ�آ�ԭ�ӵ���Χ�����Ų�ʽ ��
��2��Ԫ�آ�����γɵĻ�����ľ��������ǣ�_ _ _��
��3��Ԫ�آݡ��ĵ�һ�����ܴ�С˳���ǣ� �� ����Ԫ�ط��ű�ʾ����Ԫ�آ���Ԫ�آ��γɵ�X���ӵĿռ乹��Ϊ�� ����д��һ����N3����Ϊ�ȵ�����ķ��ӵĻ�ѧʽ ![]()
��4���ߡ�������Ԫ�����γ�һ��AB2�͵Ĺ��۷��ӣ��÷������� ���ӣ�����ԡ��Ǽ��ԡ����ݡ��ߡ� ������Ԫ��֮������ԭ�Ӹ�����1��1�����γɻ������Щ�������������������ЩԪ�صĵ��ʡ���д���ߡ�������Ԫ���γɵĻ�����Ļ�ѧʽ ������Ԫ��д��ǰ�棩��
��5��Ԫ�آ���Ԫ�����ڱ����������� ��Ԫ�أ�Ԫ�آ���һ���������γɵľ�������־�������ͼ1��ͼ2��ʾ������ͼ1��ͼ2�Ľṹ�����Ԫ��һ��ԭ�ӵȾ����������ԭ����֮��Ϊ�� ��
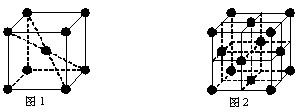
 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����



| ������I��eV�� | A | B | C | D | E | F |
| I1 | 11.3 | 14.5 | 13.6 | 5.2 | 7.6 | 6.0 |
| I2 | 24.4 | 29.6 | 35.1 | 49.3 | 15.0 | 18.8 |
| I3 | 47.9 | 47.4 | 54.9 | 71.6 | 80.1 | 28.4 |
| I4 | 64.5 | 77.5 | 77.4 | 98.9 | 109.2 | 112.0 |
| I5 | 392.1 | 97.9 | 113.9 | 138.3 | 141.3 | 153.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ������/kJ?mol-1 | I1 | I2 | I3 | I4 |
| A | 932 | 1821 | 15390 | 21771 |
| B | 738 | 1451 | 7733 | 10540 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� | |||||||||||||||||
| �� | �� | �� | �� | �� | |||||||||||||
| �� | �� | �� | |||||||||||||||
| �� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com