
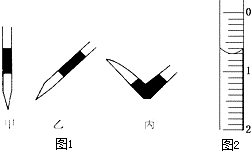
 ʹ������к͵ζ����ⶨijδ֪���ʵ���Ũ�ȵ�ϡ���ᣮ
ʹ������к͵ζ����ⶨijδ֪���ʵ���Ũ�ȵ�ϡ���ᣮ�ζ����� ʵ������/mL | 1 | 2 | 3 | 4 |
| V����Ʒ�� | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH�������ģ� | 15.80 | 15.00 | 15.02 | 14.98 |
| c(��ע)��V(��) |
| V(����) |
| c(��ע)��V(��) |
| V(����) |
| c(��ע)��V(��) |
| V(����) |
| c(��ע)��V(��) |
| V(����) |
| c(��ע)��V(��) |
| V(����) |
| c(��ע)��V(��) |
| V(����) |
| c(��ע)��V(��) |
| V(����) |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ | B������ | C������ | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
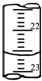 ijѧ����0.2000mol?L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��������£�
ijѧ����0.2000mol?L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��������£�| �ζ����� | ���������mL�� | ���ռ������mL�� | |
| �ζ�ǰ���� | �ζ������ | ||
| ��һ�� | 20.00 | 0.40 | 20.40 |
| �ڶ��� | 20.00 | 2.00 | 24.10 |
| ������ | 20.00 | 4.00 | 24.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ũ���� | B��Ũ���� |
| C������ͭ��Һ | D��ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
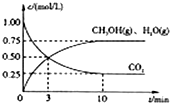 �������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�
�������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.92mol |
| B������0.46mol ��0.92mol |
| C��0.46mol |
| D����0.46mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��K+��MnO4-��Na+��Cl- |
| B��K+��Na+��NO3-��CO32- |
| C��Na+��H+��NO3-��SO42- |
| D��Al3+��Na+��Cl-��SO42- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com