
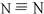 £¬µŖµÄĒā»ÆĪļ³żĮĖ°±Ęų£¬»¹ÓŠŅ»ÖÖŌŚ³£ĪĀĻĀ³ŹŅŗĢ¬µÄėĀ£¬·Ö×ÓŹ½ĪŖN2H4£¬ĒėŠ“³öėĀµÄµē×ÓŹ½
£¬µŖµÄĒā»ÆĪļ³żĮĖ°±Ęų£¬»¹ÓŠŅ»ÖÖŌŚ³£ĪĀĻĀ³ŹŅŗĢ¬µÄėĀ£¬·Ö×ÓŹ½ĪŖN2H4£¬ĒėŠ“³öėĀµÄµē×ÓŹ½ £»
£»·ÖĪö £Ø1£©µŖĘų·Ö×ÓŹĒµŖŌ×ÓŗĶµŖŌ×Ó¼äŠĪ³ÉČż¼ü£¬ėĀµÄ·Ö×ÓŹ½ĪŖN2H4£¬ŹĒµŖŌ×ÓŗĶĒāŌ×ÓŠĪ³ÉĖÄøö¹²¼Ū¼ü£¬µŖŌ×ÓŗĶµŖŌ×ÓÖ®¼äŠĪ³ÉŅ»øö¹²¼Ū¼üŠĪ³ÉµÄ¹²¼Ū»ÆŗĻĪļ£»
£Ø2£©NH3ÓėNaClO·“Ó¦æɵƵ½ėĀ£ØN2H4£©£¬NŌŖĖŲµÄ»ÆŗĻ¼ŪÉżøߣ¬¹Ź»¹Éś³ÉĀČ»ÆÄĘÓėĖ®£»
£Ø3£©“Ó×÷ĪŖČ¼ĮĻµē³ŲŹ±£¬øŗ¼«·¢ÉśŃõ»Æ·“Ó¦µÄ½Ē¶ČæÉÖŖN2H4±»Ńõ»ÆÉś³ÉN2£»ŅĄ¾Żµē¼«·“Ó¦ŗĶµē×ÓŹŲŗć¼ĘĖć£®
½ā“š ½ā£ŗ£Ø1£©µŖĘų·Ö×ÓŹĒµŖŌ×ÓŗĶµŖŌ×Ó¼äŠĪ³ÉČż¼ü£¬µŖĘųµÄ½į¹¹Ź½ĪŖ£ŗ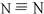 £¬ėĀµÄ·Ö×ÓŹ½ĪŖN2H4£¬ŹĒµŖŌ×ÓŗĶĒāŌ×ÓŠĪ³ÉĖÄøö¹²¼Ū¼ü£¬µŖŌ×ÓŗĶµŖŌ×ÓÖ®¼äŠĪ³ÉŅ»øö¹²¼Ū¼üŠĪ³ÉµÄ¹²¼Ū»ÆŗĻĪļ£¬µē×ÓŹ½ĪŖ£ŗ
£¬ėĀµÄ·Ö×ÓŹ½ĪŖN2H4£¬ŹĒµŖŌ×ÓŗĶĒāŌ×ÓŠĪ³ÉĖÄøö¹²¼Ū¼ü£¬µŖŌ×ÓŗĶµŖŌ×ÓÖ®¼äŠĪ³ÉŅ»øö¹²¼Ū¼üŠĪ³ÉµÄ¹²¼Ū»ÆŗĻĪļ£¬µē×ÓŹ½ĪŖ£ŗ £¬
£¬
¹Ź“š°øĪŖ£ŗ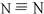 £»
£» £»
£»
£Ø2£©NH3ÓėNaClO·¢ÉśŃõ»Æ»¹Ō·“Ó¦æɵƵ½ėĀ£ØN2H4£©”¢ĀČ»ÆÄĘŗĶĖ®£¬ĖłŅŌøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ2NH3+NaClO=N2H4+NaCl+H2O£¬
¹Ź“š°øĪŖ£ŗ2NH3+NaClO=N2H4+NaCl+H2O£»
£Ø3£©ėĀŅ»æÕĘųČ¼ĮĻ¼īŠŌµē³ŲÖŠ£¬øŗ¼«ÉĻėĀŹ§µē×ÓŗĶĒāŃõøłĄė×Ó·“Ӧɜ³ÉĖ®ŗĶµŖĘų£¬µē¼«·“Ó¦Ź½ĪŖ£ŗN2H4+4OH--4e-=4H2O+N2£¬Õż¼«µē¼«·“Ó¦ĪŖ£ŗO2+2H2O+4e-=4OH-£¬ĆæÉś³É56g N2ĪļÖŹµÄĮæ=$\frac{56g}{28g/mol}$=2mol£¬Õż¼«ĻūŗÄŃõĘųĪļÖŹµÄĮæĪŖ2mol£¬ŅĄ¾Żµē×ÓŹŲŗć¼ĘĖćĻūŗıź×¼×“æöĻĀµÄŃõĘųµÄĢå»żĪŖ2mol”Į22.4L/mol=44.8L£¬
¹Ź“š°øĪŖ£ŗN2H4+4OH--4e-=4H2O+N2”ü£»44.8L£®
µćĘĄ ±¾Ģāæ¼²éøĒĖ¹¶ØĀÉ”¢µē¼«·“Ó¦Ź½µÄŹéŠ“µČÖŖŹ¶µć£¬ÕāŠ©¶¼ŹĒøßæ¼µÄČČµć£¬×¢Ņāµē¼«·“Ó¦Ź½µÄŹéŠ“ŅŖ½įŗĻµē½āÖŹČÜŅŗµÄĖį¼īŠŌ£¬ÄѶČÖŠµČ£®


 »ĘøŌ¹Ś¾üæĪæĪĮ·ĻµĮŠ“š°ø
»ĘøŌ¹Ś¾üæĪæĪĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
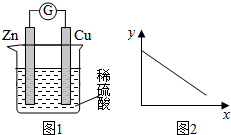
| A£® | Ķ°ōµÄÖŹĮæ | B£® | c£ØZn2+£© | C£® | c£ØH+£© | D£® | c£ØSO42-£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Č«²æ | B£® | ¢Ł¢Ś¢Ū¢Ü¢Ž | C£® | Ö»ÓŠ¢Ł¢Ś¢Ū | D£® | Ö»ÓŠ¢Ś¢Ū¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ķ¬ÅØ¶ČµÄAOHČÜŅŗŗĶH2BČÜŅŗ£¬µēĄė³Ģ¶ČĒ°Õß“óÓŚŗóÕß | |
| B£® | HB-µÄµēĄė³Ģ¶Č“óÓŚHB-µÄĖ®½ā³Ģ¶Č | |
| C£® | øĆŃĪµÄµēĄė·½³ĢŹ½ĪŖAHBØTA++H++B2- | |
| D£® | ŌŚČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”Ė³ŠņŅ»°ćĪŖ£®c£ØA+£©£¾c£ØHB-£©£¾c£ØOH-£©£¾c£ØB2-£©£¾c£ØH+£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
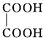
 +NaBr+H2O£»
+NaBr+H2O£»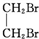 +2NaOH$”ś_{”÷}^{Ė®}$
+2NaOH$”ś_{”÷}^{Ė®}$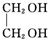 +2NaBr£»
+2NaBr£»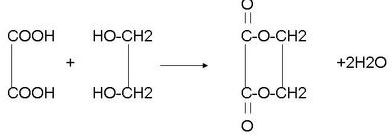 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŌŖĖŲ±ąŗÅŌŖĖŲŠŌÖŹ | ¢Ł | ¢Ś | ¢Ū | ¢Ü | ¢Ż | ¢Ž | ¢ß | ¢ą |
| Ō×Ó°ė¾¶£Ø10-10m£© | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 1.43 |
| ×īøß»ņ×īµĶ»ÆŗĻ¼Ū | +2 | +1 | +5 | +7 | +1 | +5 | +3 | |
| -2 | -3 | -1 | -3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¶Ō“óŠĶ“¬²°µÄĶāæĒ½ųŠŠµÄ”°ĪžÉüŃō¼«µÄŅõ¼«±£»¤·Ø”±£¬ŹĒÓ¦ÓĆĮĖŌµē³ŲŌĄķ | |
| B£® | ĀČ»ÆĀĮµÄČŪµć±ČŃõ»ÆĀĮµĶ£¬Ņņ“Ė¹¤ŅµÉĻ×īŗĆ²ÉÓƵē½āČŪČŚĀČ»ÆĀĮĄ“Öʱøµ„ÖŹĀĮ | |
| C£® | ¶ŌÓŚŅ±Į¶ĻńÄĘ”¢øĘ”¢Ć¾”¢ĀĮµČÕāŃł»īĘĆµÄ½šŹō£¬µē½ā·Ø¼øŗõŹĒĪØŅ»æÉŠŠµÄ¹¤Ņµ·½·Ø | |
| D£® | µē¶ĘŹ±£¬Ķس£°Ń“ż¶ĘµÄ½šŹōÖĘĘ·×÷Ņõ¼«£¬°Ń¶Ę²ć½šŹō×÷Ńō¼« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
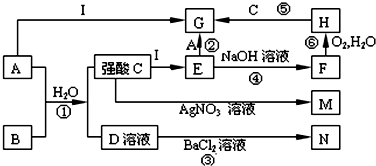
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µČÓŚ4.48L | B£® | Š”ÓŚ2.24L | C£® | “óÓŚ2.24L | D£® | µČÓŚ2.24L |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com