

| m |
| �� |
| 1 |
| 8 |
| 1 |
| 2 |
| 78 |
| NA |
| ||
| a g/cm3 |
| 4��78 |
| aNA |
| 4��78 |
| aNA |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��H+��Fe3+��I-��Cl- |
| B��Al3+��Mg2+��NO3-��Cl- |
| C��K+��Ag+��Ca2+��PO43- |
| D��NH4+��Na+��AlO2-��MnO4- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��5.6g��ƬͶ�뵽���Ũ�����У���Ƭʧȥ������Ϊ0.3NA |
| B��60g�������裨SiO2���к��е�Si-O��Ϊ4NA |
| C����״���£�3g NO��1.6gO2������������еķ�����Ϊ0.1NA |
| D����״���£�5.6L���Ȼ�̼���еķ�����Ϊ0.25 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1.0L 1.0mol/LNaAlO2ˮ��Һ�к��е���ԭ����Ϊ2NA |
| B��12gʯīϩ������ʯī���к�����Ԫ���ĸ���Ϊ0.5NA |
| C��25��ʱpH=13��NaOH��Һ�к���OH-����ĿΪ0.1NA |
| D��1mol���ǻ���1 mol������������������������Ϊ9NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
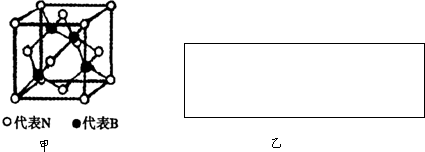
 ��������PTCԪ������Ҫ�ɷ�Ϊ���ѿ��侧��ṹ��ͼ��ʾ���ýṹ�Ǿ��д����Ե���С�ظ���λ���þ��徭X���߷����������ظ���λΪ�����壬�߳�Ϊ403.1pm������λ��ΪTi4+��ռ������λ��ΪBa2+��ռ����������λ��ΪO2-��ռ��
��������PTCԪ������Ҫ�ɷ�Ϊ���ѿ��侧��ṹ��ͼ��ʾ���ýṹ�Ǿ��д����Ե���С�ظ���λ���þ��徭X���߷����������ظ���λΪ�����壬�߳�Ϊ403.1pm������λ��ΪTi4+��ռ������λ��ΪBa2+��ռ����������λ��ΪO2-��ռ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���õ⻯�ص�����Һ��������������NO2-�Ĵ��ڣ�NO2-+I-+2H+=NO��+I2+H2O |
| B����������ˮ��Cl2+H2O=C1O-+C1-+2H+ |
| C����������������ʴ�ĸ�����Ӧ��Fe-3e-=Fe3+ |
| D����״����11.2L CO2ͨ��500mL1mol/L��NaOH��Һ��CO2+OH-=HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��H+��Na+��Cl-��CO32- |
| B��Na+��Cl-��Ba2+��SO42- |
| C��H+��K+��OH-��SO42- |
| D��H+��Al3+��Ag+��NO3- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com