����ͬѧ��ѧϰ�����ἰ���ε�ijЩ��������;���У���������ʵ��̽����
[ʵ��һ]̽��Ũ�����������
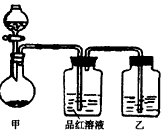
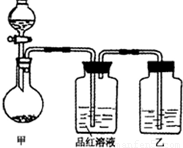
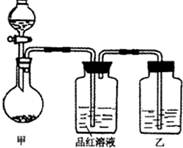
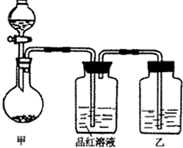
��ʵ���ҳ��õ�ҩƷ������ͼ��ʾ��ʵ��װ�ú���װ�üף����гֺͼ���װ��ʡ�ԣ�
��1����װ������ϴ�������ȱ�ݣ���ָ����______��
��2��д��װ�ü��з�Ӧ�Ļ�ѧ����ʽ��______��
��3��װ�����е��Լ���______��
[ʵ���]̽��ij���������ι����Ƿ����
��4�������������ͬѧ�������ʵ�鷽����
| ʵ����� |
Ԥ������ͽ��� |
| ______ |
______ |
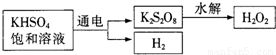
[ʵ����]��KHSO
4��ȡH
2O
2��������������
�������ϵ�֪����ҵ���õ��KHSO
4������Һ��ȡH
2O
2��ʾ��ͼ���£�
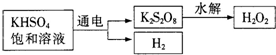
�����ô˷���ȡһ��Ũ�ȵ�H
2O
2������������ʵ��ⶨH
2O
2�����������������ӷ���ʽ��2MnO
4-+5H
2O
2+6H
+=2Mn
2++8H
2O+5O
2����
��ȡ5.00mL H
2O
2��Һ���ܶ�Ϊ1.00g/mL��������ƿ�м�ˮϡ�ͣ��ټ�ϡ�����ữ��
����0.1000mol/L KMnO
4��Һ�ζ���
����ͬ�������ζ�����������KMnO
4��Һ������ֱ�Ϊ20.00mL��19.98mL��20.02mL��
�ش��������⣺
��5����ⱥ��KHSO
4��Һʱ�������ĵ缫��ӦʽΪ______��
��6���������У������һ��KMnO
4��Һ����Һ�Ϻ�ɫ��ʧ���������ŵζ�������Mn
2+�����࣬��Һ�Ϻ�ɫ��ʧ���ʼӿ죮Mn
2+��������______��
��7��ԭH
2O
2��Һ�����ʵ���������Ϊ______��




 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д� �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
 ��2009?����һģ������ͬѧ��ѧϰ�����ἰ���ε�ijЩ��������;���У���������ʵ��̽����
��2009?����һģ������ͬѧ��ѧϰ�����ἰ���ε�ijЩ��������;���У���������ʵ��̽����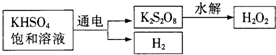
 ����ͬѧ��ѧϰ�����ἰ���ε�ijЩ��������;���У���������ʵ��̽����
����ͬѧ��ѧϰ�����ἰ���ε�ijЩ��������;���У���������ʵ��̽����