
ГҫәНәЈЛ®МбГҫ
1Ј®әЈЛ®ЦРГҫөДә¬Бҝ
ЧЬҙўБҝЈә1.28 gЎӨLЈӯ1Ј¬КАҪзЙПУР60ЈҘГҫАҙЧФУЪәЈЛ®Ј®
2Ј®әЈЛ®МбГҫөД№ӨТөБчіМ
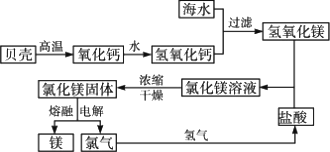
МЦВЫЈәұҙҝЗФЪЙъІъБчіМЦРЖрМṩMg2+іБөнјБөДЧчУГЈ®ЖдЦчТӘіЙ·Ц·ўЙъБЛ·ЦҪв·ҙУҰЈә
CaCO3![]() CaOЈ«CO2Ўь
CaOЈ«CO2Ўь
3Ј®ГҫөДРФЦКәНУГНҫ
(1)ГҫөДЦчТӘОпАнРФЦКЈәГЬ¶И________ЎўЗҝ¶И________Ўў»ъРөРФДЬәГөДТш°ЧЙ«ҪрКфЈ®
(2)ГҫөД»ҜС§РФЦК
ўЩУл·ЗҪрКфөҘЦК·ҙУҰ
2MgЈ«O2![]() ________
________
MgЈ«Cl2![]() ________
________
3MgЈ«N2![]() ________
________
ўЪУлCO2·ҙУҰ
2MgЈ«CO2![]() ________
________
ўЫУлЛб·ҙУҰ
2MgЈ«2HCl![]() ________
________

| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
(9·Ц)ГҫКЗТ»ЦЦЗбҪрКф,ЖдәПҪрЗҝ¶ИёЯЎў»ъРөРФДЬәГЎЈХвР©МШРФК№ҪрКфГҫіЙОӘЦЖФмЖыіөЎў·Й»ъЎў»рјэөДЦШТӘІДБП,ҙУ¶ш»сөГЎұ№ъ·АҪрКфЎұөДГАУюЎЈәЈЛ®ҝуОпЦКЦРГҫөДЕЁ¶ИҪцҙОУЪВИәНДЖ,ҫУөЪИэО»ЎЈУЙУЪ¶ФГҫөДРиЗу·ЗіЈҫЮҙу,әЬ¶аСШәЈ№ъјТ¶јҪЁУРҙуРНәЈЛ®МбГҫ№Өі§ЎЈ
ПВұнКЗУР№ШОпЦКөДK![]() :
:
| ОпЦК |
|
|
|
|
| ИЬ¶И»э | 2. | 6. | 5. | 1. |
»ШҙрПВБРОКМв:
(1)ДЬ·сЦұҪУ°СұҙҝЗСРДҘіЙ·ЫД©,ИцИләЈЛ®ЦРК№![]() ЧӘ»ҜОӘ
ЧӘ»ҜОӘ![]() іБөн,ІўҪвКНФӯТт: ЎЈ
іБөн,ІўҪвКНФӯТт: ЎЈ
(2)КөјКЙъІъЦРКЗ°С![]() ЧӘ»ҜОӘ
ЧӘ»ҜОӘ![]() іБөн,¶шІ»КЗЧӘ»ҜОӘ
іБөн,¶шІ»КЗЧӘ»ҜОӘ![]() іБөн,ЖдАнУЙКЗ ;ЗлДгНЖІвУҰҪ«ұҙҝЗҪшРРФхСщөДјУ№ӨҙҰАн ЎЈ
іБөн,ЖдАнУЙКЗ ;ЗлДгНЖІвУҰҪ«ұҙҝЗҪшРРФхСщөДјУ№ӨҙҰАн ЎЈ
(3)ДіН¬С§ФЪКөСйКТМхјюПВДЈДвёГЙъІъ№эіМ,ФЪјУКФјБКұ,ОуҪ«ҙҝјоИЬТәјУИләЈЛ®ЦР,ЛыЛјҝјБЛТ»ПВ,УЦФЪөГөҪөД»мәПМеПөЦРјУИл№эБҝөДЙХјоИЬТә,ДгҫхөГЛы (МоЎұДЬЎұ»тЎұІ»ДЬЎұ)Ҫ«![]() ЧӘ»ҜОӘ
ЧӘ»ҜОӘ![]() іБөн,АнУЙКЗ ЎЈ
іБөн,АнУЙКЗ ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2011-2012С§ДкёЈҪЁКЎЛДөШБщРЈёЯ¶юПВС§ЖЪөЪТ»ҙОБӘҝј»ҜС§КФҫн МвРНЈәМоҝХМв
(9·Ц)ГҫКЗТ»ЦЦЗбҪрКф,ЖдәПҪрЗҝ¶ИёЯЎў»ъРөРФДЬәГЎЈХвР©МШРФК№ҪрКфГҫіЙОӘЦЖФмЖыіөЎў·Й»ъЎў»рјэөДЦШТӘІДБП,ҙУ¶ш»сөГЎұ№ъ·АҪрКфЎұөДГАУюЎЈәЈЛ®ҝуОпЦКЦРГҫөДЕЁ¶ИҪцҙОУЪВИәНДЖ,ҫУөЪИэО»ЎЈУЙУЪ¶ФГҫөДРиЗу·ЗіЈҫЮҙу,әЬ¶аСШәЈ№ъјТ¶јҪЁУРҙуРНәЈЛ®МбГҫ№Өі§ЎЈ
ПВұнКЗУР№ШОпЦКөДK :
:
| ОпЦК |  |  |  |  |
| ИЬ¶И»э | 2. | 6. | 5.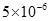 | 1. |
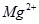 ЧӘ»ҜОӘ
ЧӘ»ҜОӘ іБөн,ІўҪвКНФӯТт: ЎЈ
іБөн,ІўҪвКНФӯТт: ЎЈ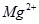 ЧӘ»ҜОӘ
ЧӘ»ҜОӘ іБөн,¶шІ»КЗЧӘ»ҜОӘ
іБөн,¶шІ»КЗЧӘ»ҜОӘ іБөн,ЖдАнУЙКЗ ;ЗлДгНЖІвУҰҪ«ұҙҝЗҪшРРФхСщөДјУ№ӨҙҰАн ЎЈ
іБөн,ЖдАнУЙКЗ ;ЗлДгНЖІвУҰҪ«ұҙҝЗҪшРРФхСщөДјУ№ӨҙҰАн ЎЈ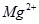 ЧӘ»ҜОӘ
ЧӘ»ҜОӘ іБөн,АнУЙКЗ ЎЈ
іБөн,АнУЙКЗ ЎЈІйҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2014ҪмәюұұКЎёЯИэЙПС§ЖЪЖЪЦРБӘҝј»ҜС§КФҫнЈЁҪвОц°жЈ© МвРНЈәМоҝХМв
әЈЛ®ЦРә¬УРј«ОӘ·бё»өДЧФИ»ЧКФҙЎЈәЬ¶аіЈјыөДҪрКфФӘЛШәН·ЗҪрКфФӘЛШЦчТӘТФјтөҘАлЧУөДРОКҪҙжФЪУЪәЈЛ®ЦРЈ¬әЈЛ®іКИхјоРФЎЈ»ШҙрПВБРОКМвЈә
әЈЛ®Мбде№эіМТ»°г·ЦИэІҪЈ¬ўЩПтЕЁЛхөДәЈЛ®ЦРНЁИлCl2Ј¬Ҫ«ЖдЦРөДBr-Сх»ҜЈ¬2Br-+Cl2=Br2+2Cl-Ј¬ФЩУГИИҝХЖшҙөіцдеЈ»ўЪИ»әуУГұҘәНМјЛбДЖИЬТәОьКХдеЈ¬деЖз»ҜОӘNaBrЎўNaBrO3Ј¬ўЫЧоәуУГБтЛбЛб»Ҝ5NaBr+NaBrO3+3H2SO4=3Na2SO4+3Br2+3H2O
ЈЁ1Ј©РҙіцөЪўЪІҪ·ҙУҰөДАлЧУ·ҪіМКҪ Ј»
ЈЁ2Ј©әЈЛ®ЕЁЛхәу»№ТӘҪшТ»ІҪҙҰАнІЕДЬНЁИлCl2Ј¬ҙҰАнөД·Ҫ·ЁКЗ Ј¬ДҝөДКЗ Ј¬ІҪЦиўЫУГБтЛб¶шІ»УГСОЛбЛб»ҜөДФӯТтҝЙДЬКЗ Ј»
ЈЁ3Ј©ОӘБЛіэИҘ№ӨТөBr2ЦРОўБҝөДCl2,ҝЙПт№ӨТөBr2ЦР Ј»
aЈ®НЁИлHBr bЈ®јУИлNa2CO3ИЬТә cЈ®јУИлNaBrИЬТә dЈ®јУИлNa2SO3ИЬТә
ЈЁ4Ј©әЈЛ®ЦЖдеөДФӯАнМеПЦБЛ»ҜС§№ӨТөЦРЎ°ё»јҜЎұЎ°·ЕҙуЎұЛјПлЎЈТФәЈЛ®ОӘФӯБПЦЖұёҪрКфГҫ·ЦИэІҪЈ¬КФРҙіцЖд·ҙУҰФӯАнЈЁУГ»ҜС§·ҪіМКҪұнКҫЈ©
Ј¬ Ј¬ ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2013ҪмёЈҪЁКЎЛДөШБщРЈёЯ¶юПВС§ЖЪөЪТ»ҙОБӘҝј»ҜС§КФҫн МвРНЈәМоҝХМв
(9·Ц)ГҫКЗТ»ЦЦЗбҪрКф,ЖдәПҪрЗҝ¶ИёЯЎў»ъРөРФДЬәГЎЈХвР©МШРФК№ҪрКфГҫіЙОӘЦЖФмЖыіөЎў·Й»ъЎў»рјэөДЦШТӘІДБП,ҙУ¶ш»сөГЎұ№ъ·АҪрКфЎұөДГАУюЎЈәЈЛ®ҝуОпЦКЦРГҫөДЕЁ¶ИҪцҙОУЪВИәНДЖ,ҫУөЪИэО»ЎЈУЙУЪ¶ФГҫөДРиЗу·ЗіЈҫЮҙу,әЬ¶аСШәЈ№ъјТ¶јҪЁУРҙуРНәЈЛ®МбГҫ№Өі§ЎЈ
ПВұнКЗУР№ШОпЦКөДK :
:
|
ОпЦК |
|
|
|
|
|
ИЬ¶И»э |
2. |
6. |
5. |
1. |
»ШҙрПВБРОКМв:
(1)ДЬ·сЦұҪУ°СұҙҝЗСРДҘіЙ·ЫД©,ИцИләЈЛ®ЦРК№ ЧӘ»ҜОӘ
ЧӘ»ҜОӘ іБөн,ІўҪвКНФӯТт:
ЎЈ
іБөн,ІўҪвКНФӯТт:
ЎЈ
(2)КөјКЙъІъЦРКЗ°С ЧӘ»ҜОӘ
ЧӘ»ҜОӘ іБөн,¶шІ»КЗЧӘ»ҜОӘ
іБөн,¶шІ»КЗЧӘ»ҜОӘ іБөн,ЖдАнУЙКЗ
;ЗлДгНЖІвУҰҪ«ұҙҝЗҪшРРФхСщөДјУ№ӨҙҰАн
ЎЈ
іБөн,ЖдАнУЙКЗ
;ЗлДгНЖІвУҰҪ«ұҙҝЗҪшРРФхСщөДјУ№ӨҙҰАн
ЎЈ
(3)ДіН¬С§ФЪКөСйКТМхјюПВДЈДвёГЙъІъ№эіМ,ФЪјУКФјБКұ,ОуҪ«ҙҝјоИЬТәјУИләЈЛ®ЦР,ЛыЛјҝјБЛТ»ПВ,УЦФЪөГөҪөД»мәПМеПөЦРјУИл№эБҝөДЙХјоИЬТә,ДгҫхөГЛы (МоЎұДЬЎұ»тЎұІ»ДЬЎұ)Ҫ« ЧӘ»ҜОӘ
ЧӘ»ҜОӘ іБөн,АнУЙКЗ
ЎЈ
іБөн,АнУЙКЗ
ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com