
��14�֣�ij��ѧ��ȤС��ͬѧչ����Ư���������ƣ�NaClO2�����о���
ʵ�����ȡNaClO2����
��֪��NaClO2������Һ���¶ȵ���38��ʱ�����ľ�����NaClO2?3H2O������38��ʱ�����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��Ba(ClO)2������ˮ��
������ͼ��ʾװ�ý���ʵ�顣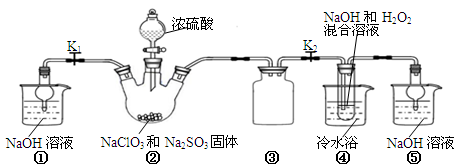
��1��װ�âٵ������� װ�â۵������� ��
��2��װ�â��в���ClO2�Ļ�ѧ����ʽΪ ��
��3����װ�âܷ�Ӧ�����Һ��þ���NaClO2�IJ�������Ϊ��
�ټ�ѹ��55�������ᾧ���ڳ��ȹ��ˣ��� ���ܵ���60�����õ���Ʒ��
��4�����ʵ���������NaClO2�����Ƿ�������Na2SO4�������������ǣ�ȡ����������������ˮ�� ��
ʵ��ⶨij����������Ʒ�Ĵ��ȡ�
�������ʵ�鷽����������ʵ�飺
��ȷ��ȡ��������������Ʒm g���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ����֪��ClO2��+ 4I��+4H+ =2H2O+2I2+Cl�����������û��Һ���100mL������Һ��
����ȡ25.00mL������Һ����ƿ�У���c mol?L-1 Na2S2O3��Һ�ζ������ζ��յ㡣�ظ�2�Σ�������ı���Һ�������ƽ��ֵΪV mL����֪��I2 +2S2O32��=2I��+S4O62������
��5���ζ���ʹ�õ�ָʾ���� ���ﵽ�ζ��յ�ʱ������Ϊ ��
��6�� ��Ʒ��NaClO2����������Ϊ ���ú�m��c��V�Ĵ���ʽ��ʾ��ʽ����NaClO2 90.5����
��1�����ն����ClO2���壬��ֹ��Ⱦ����(1��)
��ֹ����(������ȫƿ��������ȷ˵��) (1��)
��2��2NaClO3+Na2SO3+H2SO4(Ũ)==2ClO2��+2Na2SO4+H2O(2��)
��3����38�桫60�����ˮϴ��(2�֣���д��ϴ�ӡ���1��)
��4���μӼ���BaCl2��Һ�����а�ɫ�������֣�����Na2SO4�����ް�ɫ�������֣���Na2SO4�� (2�֣���һ�����1��)
��5��������Һ��1�֣�����Һ����ɫ��Ϊ��ɫ�Ұ���Ӳ���ɫ (2�֣�������ӡ���д��1)
��6�� 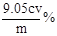 ��
�� 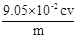 (3��)
(3��)
���������������1��װ������δ��Ӧ��ClO2��װ�âٿ�������δ��Ӧ��ClO2����ֹ�ݳ���Ⱦ������װ�â������巴Ӧ��װ����ѹǿ���ͣ�װ�â۷�ֹ������
��2��װ�â��в���ClO2�ķ�Ӧ����������������Һ��������������Ϊ�����ƣ���������ԭΪ�������ȣ���Ӧ�Ļ�ѧ����ʽΪ��2NaClO3+Na2SO3+H2SO4=2ClO2��+2Na2SO4+H2O��
��3������Һ����ȡ���壬һ����������ᾧ�����ˡ�ϴ�ӡ�����ķ�����Ϊ��ֹ��������NaClO2?3H2O��Ӧ���ȹ��ˣ�����Ŀ��Ϣ��֪��Ӧ�����¶�38�桫60�����ϴ�ӣ�����60����
��4������SO42?��Ba2+�ķ�Ӧ��������NaClO2�����Ƿ�������Na2SO4�������������ǣ�ȡ����������������ˮ���μӼ���BaCl2��Һ�����а�ɫ�������֣�����Na2SO4�����ް�ɫ�������֣���Na2SO4��
��5���������۱���ɫ������ָʾ��Ϊ��������Һ����Ӧ����ʱ���ⷴӦ��ȫ���μ����һ��Na2S2O3��Һʱ��Һ����ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��˵������ζ��յ㡣
��6������Ʒ��NaClO2����������Ϊx����NaClO2��2I2��4S2O32-
90.5g 4mol
mxg c mol?L-1��V��10-3L��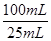
����90.5g��mxg=4mol��c mol?L-1��V��10-3L��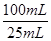 �����x=
�����x=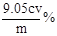 ��
�� 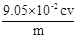 ��
��
���㣺���⿼�����ʵ��Ʊ���ʵ�鷽������������ۡ�������ԭ��Ӧ�ζ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������Ի������Һ�о�����NO3������������ԭ��Ӧ��ת����ϵ���£�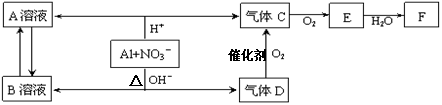
��֪������D��F��Ӧ�������Σ�����D��A��Һ��Ӧ���ɰ�ɫ������
��ش��������⣺
(1) A��B����Һ��ϲ�����ɫ��������Ӧ�����ӷ���ʽΪ ��
(2) C��E��������л���ɴ�����Ⱦ���ڴ��������£�D���Խ�C��E��ת��Ϊ������̬���ʣ�����д������һ����Ӧ�Ļ�ѧ����ʽ ��
(3)д�����ڼ�����������NO3-��Ӧ�����ӷ���ʽ ��
(4)��ȥ����C�е���������E�Ļ�ѧ������ (�û�ѧ����ʽ��ʾ)
(5)Al��NO3�������������·�Ӧ��Al�뱻��ԭ��NO3�������ʵ���֮���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��1����A����������ˮ������D�����������������������;���Ľ������ʣ���������B����Һ���ܵõ�B����B�Ļ�ѧʽ������ ����ҵ����ȡA�����ӷ���ʽ��Ϊ ��
��2����A��һ�ּ������壬�������������B������β��֮һ�����������ɫ����Ӧ�ٵĻ�ѧ����ʽΪ ��
��3����D���ȼҵ����Ҫ��Ʒ��B�����ԣ���Ӧ�ڵ����ӷ���ʽ�� ��
��4����A��C��D���dz������壬C���γ��������Ҫ���壬��Ӧ�۵Ļ�ѧ����ʽ ��
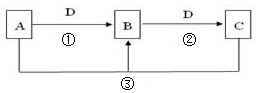
��5���ȼҵ�Ǹߺ��ܲ�ҵ��һ�ֽ�������ȼ�ϵ������ϵ��¹��տ��Խڣ��磩��30%���ϣ������ֹ�������У�������ϵĴ�����ת����ϵ����ͼ��ʾ�����еĵ缫δ��������õ�����Ĥ��ֻ����������ͨ����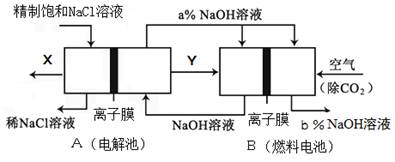
��6�����ͼ��X��Y�ֱ��� ���ѧʽ���������Ƚ�ͼʾ������������������a% b%�����������=��������
��д��ȼ�ϵ��B�и����Ϸ����ĵ缫��Ӧ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
������һ����Ҫ�Ĺ�ҵԭ�ϣ���ҵ������Ĺؼ��ǰ��Ĵ������������Ṥҵ��صĹ����в����ĵ�������Ĵ�����Ӧ��Ҳ�ǿ�ѧ�о����ȵ㡣
I��ͼ10��ͼ11�ֱ���ʵ����ģ��ϳɰ�������������װ��
��1������������ͨ��ͼ10װ�ã���װ����Ũ����������ǿ����������ٺ� ��
��2����ͼ11װ������һ��ʱ�䰱����ͨ�������ͬʱ���Ѿ����ȵIJ�˿������װ�õ���ƿ�ڣ���˿���ֺ��ȵ�ԭ���� ��д����װ���а������Ļ�ѧ����ʽ ����Ӧ��������ƿ�ڵ���Һ�к���H����OH���� ���ӡ� ���ӡ�
II�������й�������ʵ�Ľ��ͺ�������
| A��Ũ����ͨ����������ɫ���Լ�ƿ�У�˵��Ũ����ȶ� |
| B����������ϡ���ᷴӦ����Һ��dz��ɫ��˵��ϡ����������������� |
| C������Ũ������ͭм��Ӧ����ȡ����ͭ��˵��Ũ������лӷ��� |
| D������п��ϡ���ᷴӦ��ȡ������˵��ϡ�����ܽ�п�ۻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ѧij��ѧ��ȤС����ʵ������MnO2��Ũ���ᷴӦ�Ʊ�C12(����װ����ͼ��ʾ)��
��1���Ʊ�ʵ�鿪ʼʱ���ȼ��װ�������ԣ��������IJ��������� ���)��
A������ƿ�м���Ũ����
B������
C������ƿ�м���MnO2��ĩ
��2���Ʊ���Ӧ��������Ũ���½���ֹͣ��Ϊ�ⶨ��Ӧ����Һ�������Ũ�ȣ���ȤС��ͬѧ�������ʵ��̽��������
������������AgNO3��Һ��Ӧ���������ɵ�AgCl����
�ҷ������������ǡ���кͣ���ָʾ�����ķ����ⶨ
������������֪��CaCO3(����)��Ӧ������ʣ���CaCO3����
��������������Zn��Ӧ���������ɵ�H2���������Ϊ��״����
�̶����������жϺ�ʵ�飺
���ж������Ƿ���� ����ǡ����������� ��
�ڽ����ҷ���ʵ�飺ȷ��ȡ������Һϡ��һ����������Ϊ������
a����ȡ����20��00 mL������0��200 0 mol/L��NaOH��Һ������NaOH��Һ22��00 mL���ôβ������������Ũ��Ϊ mol��Lһ1��
b���ظ����ϲ������ȡƽ��ֵ���ʵ����
���жϱ�������ʵ���� (�ƫ��"����ƫС����ȷ��)��
(��֪������CaCO3��ת��Ϊ�����ܵ�MnCO3)
�ܽ��ж�����ʵ�飺װ����ͼ��ʾ(�г���������ȥ)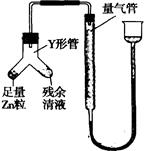
(i)ʹY�ι��еIJ�����Һ��п����Ӧ����ȷ�����ǽ� ת�Ƶ� �С�
(ii)��Ӧ��ϣ�ÿ���l���Ӷ�ȡ������������������μ�С��ֱ�����䡣���������μ�С��ԭ���� (�ų�������ʵ�������Ӱ������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ�о�ͭ��Ũ����ķ�Ӧ��ij��ѧ��ȤС���������ʵ�顣
ʵ���Ӧ����Ķ���̽����
ʵ��װ����ͼ��ʾ�����̶�װ������ȥ��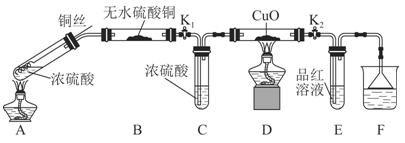
��1��A�з�Ӧ�Ļ�ѧ����ʽΪ ��
��2��F�ձ��е���Һͨ���� ��
��3��ʵ������У���֤��Ũ��������Ԫ�ص�������ǿ����Ԫ�ص�������
��
��4��ʵ�������֤��Aװ���Թ��з�Ӧ���ò����Ƿ���ͭ���ӵIJ��������� ��
��5��Ϊ˵��Ũ�����е�ˮ�Ƿ�Ӱ��Bװ��������жϣ��������һ��ʵ�顣ʵ�鷽��Ϊ ��
ʵ���Ӧ����Ķ���̽��
��6����ͭ��Ũ���ᷴӦ�Ĺ����У������к�ɫ���ʳ��֣�������������������ϡ�
����1��
| ����/mol��L��1 | ��ɫ���ʳ��ֵ��¶�/�� | ��ɫ������ʧ���¶�/�� |
| 15 | Լ150 | Լ236 |
| 16 | Լ140 | Լ250 |
| 18 | Լ120 | ����ʧ |
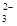 ��I2===S4O
��I2===S4O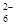 ��2I����
��2I�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����һ�Թ�NO2��ijͬѧ������±�ʵ�飬�Ծ����ܶ��ʹNO2��ˮ���ա�
��������±���
| ʵ�鲽�� | ʵ������ | ���ͣ��û�ѧ����ʽ��ʾ�� | |
| �� | ������NO2�ĵ�����ʢ��ˮ��ˮ���У���ȥ��Ƥ��������ζ��Թܡ� | �Թ�����������������ɫ��Ϊ��ɫ��Һ���������Թܸ߶ȵ�����������ܿڣ����� | |
| �� | | | |
| �� | �ظ�����ڵIJ������Σ�ֱ��Һ����������Թܡ� | �Թ��ڳ�����ɫҺ�塣 | �ܷ�Ӧ����ʽ�� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
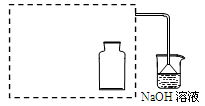
��16�֣�ij�о�С�����о�̼��Ũ����ķ�Ӧ����ʵ��������¡�
| ���� | ���� |
| a���ø���ྻ���ձ�ȡԼ10 mLŨ���ᣬ���ȡ� | |
| b����С���պ��ľ̿Ѹ�������ȵ�Ũ�����С� | ���ȵ�ľ̿���ȵ�Ũ����Ӵ��������ҷ�Ӧ��ͬʱ�д�������ɫ���������Һ����ľ̿Ѹ��ȼ�գ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
CaCO3�㷺��������Ȼ�磬��һ����Ҫ�Ļ���ԭ�ϡ�����ʯ��Ҫ�ɷ�ΪCaCO3�������������ĺ����ʵ�����ô���ʯ��ϡ���ᷴӦ�Ʊ�CO2���塣����װ�ÿ�����CO2������ᴿ���
���������գ�
(1)��Ũ��������1��1(�����)��ϡ����(Լ6 mol��L��1)��Ӧѡ�õ������� ��
a���ձ� b�������� c����Ͳ d������ƿ
(2)����װ���У�A�� ��NaHCO3��Һ�������� ��
(3)����װ���У�B������ �������ʵ��õ�������ⶨCO2����Է������������B����ʧЧ���ⶨ��� (�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
(4)һ���Է�����ʯ��(������)��CaCO3��ʳ���е��ܳ��������۷���������ָ��֮һ���ⶨ�ܳ�������Ҫʵ�鲽��������£�
���顢���ء������ܽ�����ˡ�������ɡ���ȴ�����ء�����
Ϊ�˽�ʯ����̼����ܳ���Ӧѡ�õ��Լ��� ��
a���Ȼ�����Һ b��ϡ���� c��ϡ���� d��������
(5)���ܳ����ⶨʵ���У�Ϊ�˻��ʯ����̼��Ƶ�����ܳ�����Ӧ���ܳ� �����ܳ� ��
(6)�����ⶨʵ���У����� ��˵����Ʒ�Ѿ����ء�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com