
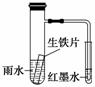
 ��ͼװ���У�С�Թ���Ϊ��īˮ����֧�Թ���ʢ��pH��4���õ���ˮ������Ƭ��ʵ��ʱ�۲쵽����ʼʱ������Һ���½���һ��ʱ�����Һ��������Ը���С�Թ���Һ�档����˵����ȷ���� (����)
��ͼװ���У�С�Թ���Ϊ��īˮ����֧�Թ���ʢ��pH��4���õ���ˮ������Ƭ��ʵ��ʱ�۲쵽����ʼʱ������Һ���½���һ��ʱ�����Һ��������Ը���С�Թ���Һ�档����˵����ȷ���� (����)
A������Ƭ�е�̼��ԭ��ص�������������ԭ��Ӧ
B����ˮ���Խ�ǿ������Ƭ���������ⸯʴ
C��īˮ����ʱ��̼�缫��ӦʽΪO2��2H2O��4e��===4OH��
D����֧�Թ�����ҺpH��С
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�������£���һ�ܱ������н�1.0 mol��L��1 N2��3.0 mol��L��1 H2�ϳɰ�����Ӧ��2 sʱ���NH3��Ũ��Ϊ0.8 mol��L��1�����ð���Ũ�ȵ���������ʾ�÷�Ӧ�ķ�Ӧ����ʱ���÷�Ӧ�ķ�Ӧ����Ϊ(����)
A��0.2 mol��L��1��s��1�� B��0.4 mol��L��1��s��1 C��0.6 mol��L��1��s��1 D��0.8 mol��L��1��s��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E��F���ֶ�����Ԫ�أ�AԪ�ص�������ɫ��ӦΪ��ɫ��5.8 g B����������ǡ������100 mL 2 mol��L��1������ȫ��Ӧ��Bԭ�Ӻ�������������������ȡ�Fԭ������������F2�ڻ���ɫ����C2��ȼ�ղ�����ɫ���档DԪ��ԭ�ӵ������������Ǵ�����������3������EԪ�صĻ�����������ࡣ�������������ش�
��1��E��Ԫ�ط���Ϊ ��
��2��A��D�γɵ���ɫ������A2D2�������ʽΪ ��
��3��C2��ADF��Һ��Ӧ�����ӷ���ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�������õ�ⷨ����������ˮ�������������������������������ʢ�ź�����ˮ��ԭ��ʾ��ͼ���£�����˵������ȷ���� (����)
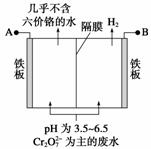
A��AΪ��Դ����
B����������Һ�з�����������ԭ��ӦΪ
Cr2O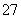 ��6Fe2����14H��===2Cr3����6Fe3��
��6Fe2����14H��===2Cr3����6Fe3�� ��7H2O
��7H2O
C��������������ҺpH����
D����������������ܽ⣬���ռ���H2 13.44 L(��״��)ʱ����0.1 mol Cr2O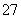 ����ԭ
����ԭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)���н�������Խ��Խ���ױ���ʴ (����)
(2)�����������ں����������ⸯʴԭ��һ�� (����)
(3)���ﻷ���½���������ʴ (����)
(4)Al��Fe��Cu�ڳ�ʪ�Ŀ����и�ʴ������������ (����)
(5)���������绯ѧ��ʴʱ��������ʧȥ��������Fe3�� (����)
(6)�ڽ������渲�DZ����㣬���������������ȫʧȥ�˶Խ����ı�������(����)
(7)��ӵ����������������������˵��أ���������������������ԭ��ء����߾�����Ч�ر������������ױ���ʴ (����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��ǿ������ʴ�ԣ�����Ǧ����Ϊ���Դ����Al��������Pb�����������ϡ���ᣬʹ�����������Ĥ����Ӧԭ�����£�
��أ�Pb(s)��PbO2(s)��2H2SO4(aq)= ==2PbSO4(s)��2H2O(l)
==2PbSO4(s)��2H2O(l)
���أ�2Al��3H2O���,Al2O3��3H2��
�������У������ж���ȷ���� (����)
| ��� | ���� | |
| A | H������Pb�缫 | H������Pb�缫 |
| B | ÿ����3 mol Pb | ����2 mol Al2O3 |
| C | ������PbO2��4H����2e��===Pb2����2H2O | ������2Al��3H2O��6e��===Al2O3��6H�� |
| D |
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڵ��ԭ����������ȷ���� (����)
A��Ϊ��ֹ�ִ���ʴ�����ִ�����������Դ�ĸ�������
B����пƬ�϶�ͭ�����Ȼ�п��Һ�����Һ
C������Ȼ�����Һ�Ʊ�����������ʱ������������
D���õ�ⷨ����ͭʱ���������Ͽ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������һ����Ϊͬϵ����ǣ� ��
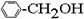
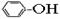 ��CH4 ��C3H6 ��C8H18 ��CH2=CH-CH2CH3 �� ��
��CH4 ��C3H6 ��C8H18 ��CH2=CH-CH2CH3 �� ��
A���٢� B���ڢ� C���ݢ� D���٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������(Na2S2O5)�dz��õ�ʳƷ��������֮һ��ij�о�С���������ʵ�飺
ʵ��һ ���������Ƶ���ȡ
������ͼװ��(ʵ��ǰ�ѳ���װ���ڵĿ���)��ȡNa2S2O5��װ��II����Na2S2O5���������������ķ�ӦΪ��
Na2SO3��SO2��Na2S2O5
��1��װ��I�в�������Ļ�ѧ����ʽΪ ��
��2��Ҫ��װ��II�л���������ľ��壬�ɲ�ȡ�ķ��뷽���� ��
��3��װ��III���ڴ���β������ѡ�õ������װ��(�г���������ȥ)Ϊ (�����)��
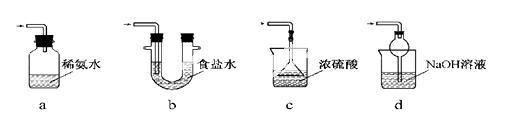
ʵ��� ���������Ƶ�����
Na2S2O5����ˮ������NaHSO3��
��4��֤��NaHSO3��Һ��HSO3�� �ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽���� (�����)��
a���ⶨ��Һ��pH b������Ba(OH)2��Һ c���������� d������Ʒ����Һ
e������ɫʯ����ֽ���
��5������Na2S2O5�����ڿ������ѱ�������ʵ�鷽���� ��
ʵ���� ���Ѿ��п��������������IJⶨ
��6�����ѾƳ���Na2S2O5�������������ⶨij���Ѿ��п��������IJ�����(������SO2����)�ķ������£�

(��֪���ζ�ʱ��Ӧ�Ļ�ѧ����ʽΪSO2��I2��2H2O��H2SO4��2HI)
�ٰ���������ʵ�飬���ı�I2��Һ25.00 mL���ô�ʵ������Ʒ�п��������IJ�����(������SO2����)Ϊ g·L��1��
��������ʵ������У����в���HI���������������ý�� (�ƫ�ߡ���ƫ�͡����䡱)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com