
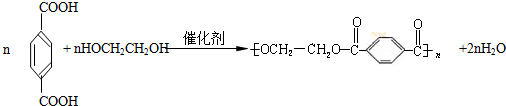
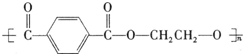
��11�֣�PET�������ϲ������ĺϳ���ά����ṹ��ʽΪ��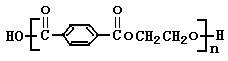
������ʯ�Ͳ�Ʒ�Զ��ױ������ϩ��DΪԭ�ϣ���ƺϳ�PET�Ĺ�ҵ������������ͼ��ʾ����Ӧ�в�������Ӧ�P����δ�г�����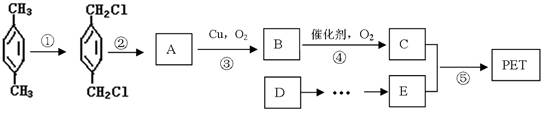
��ش��������⣺
��1��������Ӧ��ʱ�������ķ�Ӧ�P��Ӧ������ �� ��������Ӧ��ʱ�������ķ�Ӧ�P��Ӧ������ �� ��
��2���л���A�ж���ͬ���칹�壬д����������������A��һ��ͬ���칹��X�Ľṹ��ʽ ��
����X�е���FeCl3��Һ����Һ����ɫ����X�ķ��ӽṹ��ֻ��һ��������1molX�ֱ��������Ľ����ơ�NaOH��Һ��Ӧ������n(Na):n(NaOH)=2:1��
��3��д����Ӧ�ݵĻ�ѧ����ʽ ���䷴Ӧ������ ��
��4����D��E����ҵ��һ��ͨ��������Ӧ����ɡ����˴�ԭ��������100%�ĽǶ����ͨ��D��ij����һ���ϳ�E��������Ļ�ѧʽ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�




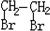
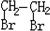





�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��������ʯ�Ͳ�Ʒ�Զ��ױ������ϩ��DΪԭ�ϣ���ƺϳ�PET�Ĺ�ҵ�����������£���Ӧ�в�������Ӧ�P����δ��ʾ����
��������ʯ�Ͳ�Ʒ�Զ��ױ������ϩ��DΪԭ�ϣ���ƺϳ�PET�Ĺ�ҵ�����������£���Ӧ�в�������Ӧ�P����δ��ʾ����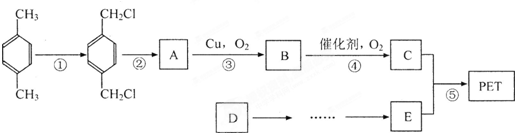
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ѧѡ��5���л���ѧ������
PET�������ϲ������ĺϳ���ά����ṹ��ʽΪ��
����ú�ĸ����ƷA��FΪԭ���Ʊ�PET��������������ͼ��ʾ������AΪ������̼Ԫ�ص���������Ϊ90.6%�����������ܶ��ǿ����ܶȵ�3.66������ʹ���Ը��������Һ��ɫ��������ʹ��ˮ��ɫ��M����������ԭ�ӹ�ƽ�档
��ش��������⣺
��1��ָ�����з�Ӧ���ͣ��� ��
��2��ָ����Ӧ�ٵķ�Ӧ������
��3��д���л���Ľṹ��ʽ��A�� ��N�� ��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ�� ��Ӧ�� ��Ӧ��
��5��P��һ��ͬϵ��X����ʽΪC3H8O2���ں˴Ź�������ͼ�г��������źŷ壬����ǿ��֮��Ϊ2�U1�U1����X�Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ��������ѧУ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
PET�������ϲ������ĺϳ���ά����ṹ��ʽΪ

ԭ�ϣ���ƺϳ�PET�Ĺ�ҵ������������(��Ӧ�в�������Ӧ�P����δ��ʾ)��

��ش��������⣺
(1)������Ӧ�������ķ�Ӧ�P��������_____________��_____________��
������Ӧ�ڵķ�Ӧ�P��Ӧ������_____________��_____________��
(2)���̢ݵķ�Ӧ������_____________��
(3)д����Ӧ�۵Ļ�ѧ����ʽ_______________________________________��
(4)�л���A�ж���ͬ���칹�壬д����������������A��һ��ͬ���칹��X�Ľṹ��ʽ��____��
����X�е���FeCl3��Һ����ɫ����X���ӽṹ��ֻ��һ��������1molX�ֱ��������Ľ����ơ�NaOH��Һ��Ӧ������n(Na)��n(NaOH)=2��1��
(5)��D��E����ҵ��һ��ͨ��������Ӧ����ɡ����˴�ԭ��������100���ĽǶ����ͨ��D��ij��������һ���ϳ�E����������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ������11��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
(15��)PET�������ϲ������ĺϳ���ά����ṹ��ʽΪ��
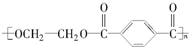
����ú�ĸ����ƷA��FΪԭ���Ʊ�PET�������Ĺ���������ͼ��ʾ������AΪ������̼Ԫ�ص���������Ϊ90.6%���������ܶ��ǿ����ܶȵ�3.66��������ʹ���Ը��������Һ��ɫ��������ʹ��ˮ��ɫ��M����������ԭ�ӹ�ƽ�档
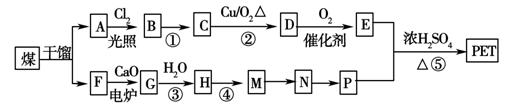
��ش��������⣺
(1)A�ķ���ʽΪ��________�����з�Ӧ����Ϊ��M�D��N________����Ӧ��________��
(2)��Ӧ�ٵķ�Ӧ����Ϊ��________��A������Ϊ________��
(3)д���л���A����һ�ȴ���Ľṹ��ʽ��
________________________________________________________________________��
(4)д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ�ۣ�_______________________________________________________________��
D��������������ͭ����Һ��У�____________________________________________��
(5)P��һ��ͬϵ��X�ķ���ʽΪC3H8O2���ں˴Ź�������ͼ�г��������źŷ壬����ǿ��֮��Ϊ2��1��1����X�Ľṹ��ʽΪ_________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com